Abstract
Environmental pollution has become a global issue due to continuing anthropogenic activities that result in the production of enormous amounts of waste and the subsequent release of hazardous trace metals. The increasing levels of trace metals in the environment must be monitored regularly and reduced to prevent contamination of food chain. Numerous conventional technologies that are widely used for the removal of trace metals from environmental matrices have many drawbacks. Currently, the preferred method to remove trace metal ions is the adsorption process, which normally uses adsorbents. This review investigated the applications of coal fly ash (CFA) as a cost-effective adsorbent and the role it plays in the improved properties of nanomaterials that are used for treatment of trace metals in water. The use of CFA and its role in chemical modification processes results to high removal efficiency of trace metals. CFA is a by-product of coal combustion which is available in abundance and therefore its use is not only beneficial in water treatment processes, but also reduce the burden of solid waste disposal.
Keywords: Environmental matrices, Adsorbents, Coal fly ash, Nanomaterials
1. Introduction
Coal mining and anthropogenic activities are still the main sources of environmental pollution due to the production of tailings, solid and liquid wastes characterized by various toxic pollutants, including trace metals [1]. For example, coal-based thermal power plants contribute significantly to environmental pollution through the generation of coal fly ash (CFA), which requires large areas of land for disposal. This results in the loss of vegetation, and in certain cases, a threat to wetland ecosystems and the people who live nearby, by leaving behind a negative ecological impact [2]. Moreover, through leachate and wind movements from CFA dams, the release of organic and inorganic pollutants such as metals and their derivatives may occur [[3], [4], [5], [6]]. In the past few years, there has been a cumulative global public health and ecological alarm linked to environmental contamination by trace metals [6,7]. Environmental contamination with trace metals is a major concern in point source locations like smelters and foundries, metal-based industrial and mining activities [7]. Evaporation of some metals from water bodies, soil and groundwater, soil erosion of metal ions, sediment re-suspension, leaching of trace metals, air deposition, and metal corrosion can all lead to the environmental pollution [8,9]. Natural processes such as volcanic eruptions and weathering processes have also been reported to highly contribute to the release of trace metals into the environment [6,10]. With the hypothesis that toxicity and heaviness of metals are interrelated, according to Tchounwou et al. [7], metalloids like arsenic, which can induce toxicity at low exposure levels are also considered as trace metals. Trace metals have negative effects on human health as well as on food chain and marine ecosystem [7,11,12]. Because trace metals are found in diverse environmental matrices in trace amounts, and therefore are regarded as trace elements [13]. Trace metals are non-biodegradable and carcinogenic (chromium, arsenic, beryllium, nickel cadmium, etc.), hence their presence in water in higher concentrations could induce critical health problems to biota [[14], [15], [16]]. Physical parameters such as phase association, temperature, sequestration, and adsorption have an impact on their bioavailability. It is also impacted by lipid octanol/water partition coefficients, solubility, chemical variables, and complexation kinetics that affect speciation at thermodynamic equilibrium. Due to continuing mining, industrial and agricultural activities that leave behind hazardous substances, the synthesis of environmentally-friendly, efficient, and cost-effective methods of trace metal removal from the environment is urgently required [10].
Various techniques have been applied to remove or reduce trace metals from different environmental matrices [17]. Methods such as membrane filtration, adsorption, precipitation, electrochemical, reverse osmosis, solvent extraction, electrodialysis, photocatalytic degradation, flocculation, flotation, ion-exchange, coagulation, and advanced oxidation processes are mostly used [14,15,[18], [19], [20], [21]]. The adsorption process presents advantages including effectiveness, operation simplicity and cost-effectiveness over other treatment methods. Adsorption is highly favored due to its potential to remove both organic and inorganic pollutants, even at low levels from wastewater. It is one of the most efficient methods for the removal of metals due to its low-cost, high efficiency, easy operation, and its performance, which is mainly dependent on the type of adsorbent used. CFA is gaining much interest as a low-cost and effective adsorbent or as a starting material to prepare other adsorbents materials. Various studies were conducted on the development of efficient and environmentally-friendly adsorbents from CFA [[22], [23], [24]].
1.1. Physical and chemical properties of CFA
A complete understanding of the physical and chemical properties of CFA, such as mineralogy, composition, morphology, and surface chemistry, renders a clear frame for exploring the possibility of its new applications. The chemical composition of CFA depends on the feed coal, whether it is anthracite, bituminous, sub-bituminous or lignite, which implies the content of metal oxides, sulphur and loss of ignition (LOI). CFA is divided into two major classes, class C and class F depending on its origin and chemical composition [25]. Class F mostly originates from bituminous and anthracite coals, and it has higher LOI than class C. It also has pozzolanic properties, which make it ideal for usage in the construction industry for manufacturing cement, bricks, and road beds [26,27]. Class C CFA is normally produced from sub-bituminous and lignite coals and has both pozzolanic and cementitious properties. The amount of unburned carbon in the CFA contributes to its physical appearance (from dark brown to grey), the darker the color of CFA (e.g.: anthracite and bituminous CFA), the higher the carbon content and their particle size distribution is close to that of silt (<0.075 mm) [28]. CFA particles have a spherical shape, either hollow or solid and amorphous depending on the coal type. CFA generation is highly linked to coal mining activities and has become a major pollutant of environmental matrices in different ways such as release of dust, particulate matter, mine drainage and coal residue dumping.
1.2. The use of CFA in the removal of trace metals from water
CFA poses an environmental challenge since it requires a huge space to be disposed of, and its application as an adsorbent can contribute significantly to pollution reduction. Trace metals in wastewater occur from various activities including agriculture, mining and industrial development. Due to their inherent toxicity, vast origins, persistence, and non-degradability nature, among other reasons, trace metals can be exceedingly detrimental to humans, animals, plants, and the environment [1]. To remediate trace metal-containing wastewaters, three types of methods are available: physical, chemical and biological. Electrochemical treatment technologies, ion-exchange and chemical precipitation are some of the chemical treatments that are currently used [29]. Biological methods employ microorganisms and adsorbents. Adsorption which can be physical or chemical is amongst the methods that use low-cost nanomaterials (activated carbon, CFA or CFA-based adsorbents and other cheap adsorbents from recycled waste raw materials) to decontaminate the environment [1,15,19,30]. This method has a wide range of advantages such as the generation of a small amount of sludge, reusability of the adsorbents, a simple design to operate, availability of raw materials, cost-effectiveness, automaticity, reasonable technical maturity, eco-friendly technique, and low-cost synthesized adsorbents that are commercially supplied [14,31,32]. The adsorption process can utilize adsorbents produced from different waste raw materials such as waste tire-activated carbon, polymers and fruit peels due to the characteristics of lignin, pectin, cellulose and hemicellulose which provide metal binding sites facilitating their removal [[32], [33], [34], [35], [36]]. The adsorbent is an integral part that captures pollutants onto itself and adsorbents can either be conventional or non-conventional. Examples of conventional adsorbents include ion-exchange resins (porous crosslinked polymers, non-porous resins and polymeric organic resins), commercial activated carbons (peat, wood, coals, coconut shells) and inorganic materials (activated alumina, molecular sieves, zeolites, and silica gel) [37,38]. The non-conventional adsorbents are of natural origin or sourced from industrial waste by-products such as CFA and activated carbons obtained from industrial by-products (red mud, metal hydroxide sludge, CFA, and sludge) and agricultural solid wastes (bark, sawdust and solid wastes), natural materials (clays, siliceous and inorganic materials), industrial by-products such as red mud, bio-sorbents such as chitosan and chitin, and miscellaneous adsorbents such as hydrogels, cotton waste, calixarenes, and cucurbituril [37,[39], [40], [41]]. Amongst them, CFA is a technologically feasible low-cost and effective adsorbent to remove trace metals from wastewater as it is highly available and has high content of metal oxides [42,43]. The adsorption process also uses polymers and biopolymers or sorbents to enhance the activity of nanofillers and adsorbents [42,44]. The interest in nanomaterials is gaining momentum because they are regarded as effective adsorbents for wastewater treatments due to their abundant reactive sites, large specific surface areas, nanostructure nature, and small particle sizes. They have high porosity, good pore channel connectivity, facile tunability, excellent biocompatibility, and easily scalable fabrication [[45], [46], [47]]. Adsorption depends on the surface area of the adsorbent, permeability, available sites, and types of interactions and does not change the morphology due to the regeneration capacity of the adsorbents [11,41,48]. Low-cost adsorbents can be obtained from industrial by-products and their huge specific surface area sets them apart from traditional adsorbents in terms of adsorption rate. Adsorbents can be based on nanoparticles and according to Gacem, Telli, & Khelil [41], they offer enormous potential for cleaning processes and are more active and quicker to remove different pollutants and trace metals with a relatively high density from wastewaters. However, the adsorption of trace metals using CFA as a low-cost adsorbent has been extensively explored [49].
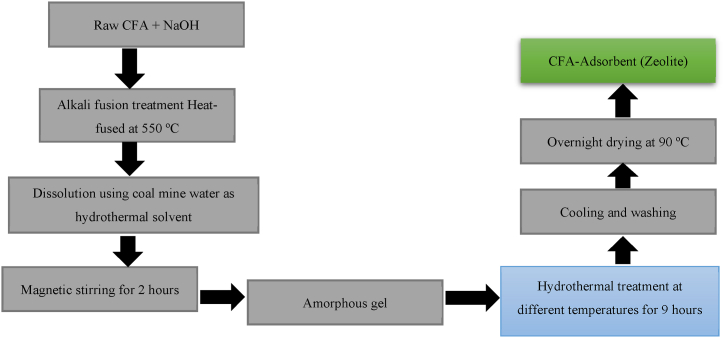
CFA can reduce environmental pollution through easy processing due to its high porosity, appropriate pore size, high surface area, and other properties (such as high percentage of CaO and unburned carbon left in the CFA particles). According to Wilkins et al. [50], the use of CFA has gained much attention due to its increased adsorption capability and therefore also used to improve the adsorption capacity of other commercially available adsorbents. For example, zeolite-A can be synthesized from CFA using hydrothermal conversion with tap water or mine water as a dissolution solvent (see Fig. 1), microwave irradiation, ultrasound and fusion techniques. When compared to untreated CFA, modified-CFA demonstrated considerably higher levels of trace metal adsorption capacity [51].
Fig. 1.
Preparation of zeolite from CFA using hydrothermal approach.
The removal efficiency of trace metals from wastewater is influenced by the choice of an adsorbent [15]. The trace metal ions that have received the most interest include Cd, Cu, Pb, Zn, Mn, Cr, and Ni [52]. For example, Ankrah et al. [53,54] effectively removed trace metals from industrial effluents using CFA as an adsorbent. The adsorption of trace metals is enhanced by higher amount of aluminium and silica oxides available in the CFA [55,56]. It is however important to monitor the pH, as studies have shown that the pH of the aqueous solution directly affects the adsorption of metal ions due to fluctuations in the surface charge of adsorbent and ionization level. For instance, significant Cu (II) and Zn (II) ion adsorption is possible with CFA at pH 8 [54]. The use of CFA to adsorb Ni (II) and Cr (III and VI) ions from wastewater has also been investigated in a few studies [57,58]. According to Rao et al. [57], the highest efficiency was reported at lower pH values and Cr (VI) concentrations were lowered from wastewater using CFA. Adsorption is also highly influenced by other factors such as temperature, contact time, and an adsorbent dosage or mass. Moreover, the concentration of metal in the aqueous solution, temperature, pH and particle size all affected the percentage of adsorbate removal by CFA [58,59]. Cr (VI) adsorption on CFA was observed to be endothermic, and kinetic studies showed that the overall adsorption rate adhered to the pseudo-second order [60]. At low initial Cr (VI) concentrations, film diffusion effects assisted in limiting the total rate of adsorption; but at higher initial Cr (VI) concentrations, pore diffusion became more significant. At 20 °C, the adsorption capacity was high, with a reported value of 6.82 mg. g−1 [61]. Furthermore, CFA materials with different carbon and mineral contents (oxides) were used as adsorbents to investigate the roles of adsorption in the removal of aqueous Cu (II) [52,62]. Copper (II) reduction in wastewater was significantly influenced by carbon content in CFA [12]. Aigbe et al. [52] studied the selective adsorption of various metal ions and the study assessed the potential of two CFA samples based on their chemical composition. The CFA sample with high amount of carbon and oxides was found to be able to selectively adsorb metals from aquatic systems [12,63,64]. Dynamic column experiments were used to assess the saturation capabilities of sorbents by monitoring breakthrough volumes of trace metal solutions [50]. According to Zhao et al. [65], the order of insolubility for the corresponding metal hydroxides determined the sorption sequence as Cu > Pb > Cd. When CFA was impregnated with Fe and Al, the adsorption of Ni (II), Zn (II) and Cr (VI) was highly improved [66]. Using enthalpy change data, it was determined that adsorption was exothermic for Ni (II) and endothermic for Zn (II) [51,65]. Hussain et al. [51] found that the alkaline character of the CFA neutralizes the metal solution, which is linked to the removal efficiency. Notably, CFA is able to adsorb trace metals without being impacted by high ionic strength or notable amounts of Cl and Ca [67]. When the concentration of calcium (CaO) increases, CFA adsorption efficiency increases as well [27]. The effect of CFA treatment on the concentrations of trace metals and toxicity in wastewater treated by a municipal wastewater treatment facility was examined by Ajorloo et al. [68]. The amounts of Pb and Cu were significantly reduced after 4 h, lowering the toxicities of these elements. Adsorption of Cu and Pb by CFA is an effective means for lowering the toxicities of these elements in wastewater [69]. A study showed that up to 90–95 % of Cu and Zn are removed by the generated adsorbent in batch and column studies. When the adsorption of both metal ions increased with temperature, it was demonstrated that the adsorption process was exothermic [67]. The removal efficiency of metals is influenced by the concentration of the metals present in wastewater. At an ideal pH of 4.0, Zn removal percentage was effective at low concentrations and 60–65 % removal efficiency was achieved at higher concentrations of the metal within 6 to 8 h equilibration period [70,71]. At higher concentrations, removal occurred through particle diffusion; at lower concentrations, it occurred through the film diffusion process [61]. Umejuru, Prabakaran, & Pillay [72] demonstrated the effectiveness of CFA modified with graphene and polyaniline (CFA/GO/PANI) nanocomposites for adsorption of Cr. Nanoparticles of iron oxide are recognized as effective adsorbents due to low-cost, recyclable nature and magnetic properties and these materials were successfully recovered from CFA, this highlighting its advantage as a valuable resource [73].
1.3. Preparation of CFA-based nanocomposite materials or adsorbents
In recent years, the use of CFA has received a lot of attention due to its potential to reduce the environmental burden of solid waste disposal, while contributing to economic gain [74,75]. CFA can work independently as an adsorbent as it contains nanosized particles (1–100 nm) with higher reactivity and large surface area and can also be incorporated into other adsorbent materials to improve their effectiveness [76]. The high efficiency of CFA-based adsorbents is also attributed to their high surface area which can be as high 1000 m2 g−1, their alkaline nature and capacity for adsorption/co-precipitation of elemental ions.
Most of the economical and efficient CFA-based adsorbents are generally produced using conventional methods such as impregnation with non-metallic or metallic compounds and chemical modification through basic and acidic treatment [77]. The tight wrapping of adsorbents on the CFA surface normally reduces the recombination of holes and electron charge carriers [78]. In addition, several other methods such as fusion, ultrasonic, hydrothermal treatment, sol-gel, microwave irradiation, and a combination of some of them such as sol-gel-hydrothermal method are generally used to produce various adsorbents and nanomaterials (photocatalysts, zeolites, geopolymers, and catalysts) from CFA [72,73,77,79,80]. Although CFA is cheap and has been evaluated as an effective adsorbent, there are some limitations in its application which require chemical modification. The limitations are attributed to its fineness property, low absorption capacity and content of hazardous trace metals. For example, Al-Zboon et al. [81] and Huang et al. [82] modified the chemical properties of CFA with sodium hydroxide and the results revealed its enhanced adsorption properties due to chemical modification. Other CFA-based materials tested to increase the adsorption capacity of CFA include modified zeolites, CFA/C HNCPs, TiO2/CFA, ZnO/CFA, CFA-decorated with tungsten oxide-graphene oxide, Mg(OH)2/calcined CFA nanocomposite and polyethyleneimine (PEI)/CFA [4,12,45,75,[83], [84], [85], [86]]. For examples, Tauanov et al. [85] and Mofulatsi et al. [86] have also used a manganese coated zeolite to adsorb Cu (II) and Pb (II) ions and showed that the coating makes the adsorbent more efficient.
2. Beneficiation of CFA in the synthesis of adsorbents or nanocomposite materials, their characterization and applications
Characterization is an important step to identify the properties of the product and is mainly carried out for physical, chemical, morphological and mineralogical analysis purposes [77,87]. Different instruments and techniques are used to characterize the synthesized adsorbents and nanocomposite materials depending on the application or remediation process or nature of these materials [46,73,88,89]. Different parameters such as dispersion, functional group of nanoparticles, morphological shape, features, elemental composition, crystallinity, internal or surface morphology, d-spacing, amorphous nature, surface area and the structure, can be identified. The double beam spectrophotometer can be used to analyse the dispersion of final purified silica nanoparticles (SiNPs), while the Fourier transform-infrared spectroscopy (FT-IR) is used to identify the functional groups [72].
The morphological shape and features of SiNPs and residues were determined using field emission scanning electron microscope (FESEM) while their elemental composition was analyzed using electron diffraction spectroscopy (EDS) attached to the FESEM [72,73]. Yadav et al. [73] also used X-Ray diffraction (XRD) and transmission electron microscope (TEM) to determine the crystallinity and the internal morphology of these nanoparticles. Moreover, high-resolution TEM (H-RTEM) was used to obtain the d-spacing of the SiNPs, while the scattering area electron diffraction (SAED) was used to identify the amorphous nature or crystallinity of the nanoparticles. In another study, Umejuru, Prabakaran, & Pillay [72] conducted a study on CFA/GO/PANI nanocomposites for effective adsorption of Cr (VI) and its reuse for photocatalysis. The surface area of CFA/GO/PANI nanocomposite was identified through Braunauer-Emmett-Teller (BET) analysis and the functional groups were also identified using FT-IR. Angaru et al. [75] conducted a study on the synthesis of economical feasible CFA-based zeolite-supported nanozerovalent iron and nickel (nZVI/Ni@FZA) bimetallic composite for potential removal of trace metals from industrial effluents. The authors used SEM-EDS, BET, XRD, and X-ray photoelectron spectroscopy (XPS) to characterize the structure of the zeolite as well as their formed composite material. Imoisili, Nwanna, & Jen [79] characterized the synthesized silica nanoparticles from a South African CFA using XRD for elemental composition and crystallinity was determined by X-Ray fluorescence (XRF) spectrometer and XRD. Furthermore, Wen et al. [45] used BET, FT-IR, SEM/SEM-EDS and XPS to characterize the synthesized bio-sorbent composite (chitosan-coated with CFA utilized to adsorb chromium (VI) from aqueous solutions. Similarly, Adamczuk, & Kołodyńska [80] used the same techniques in addition to XRD for the characterization of the CFA-coated with chitosan.
The application of CFA as an adsorbent material is attributed to the inorganic or organic nano-sized particles (ultrafine glassy spheres, cenospheres, nanotubes, and carbonaceous nanospheres) with surface area as high as 1000 m2. g−1 that can adsorb trace metals from wastewater and can be incorporated into other materials or as a synthesized composite to enhance its removal efficiency [49,90,91].
CFA-based adsorbent materials that have been applied to remove trace metals from industrial effluents, water, CFA slurry, and wastewater [4,12,45,72,75,79,92] are illustrated in Table 1.
Table 1.
Examples of research on some CFA-based adsorbents and nanoparticles.
| Parameters | Adsorbent or nanocomposite materials |
||||||
|---|---|---|---|---|---|---|---|
| CFA-based zeolite-supported nanozerovalent Fe and Ni bimetallic composites | Zeolite LTA (CFA-ZA) | CFA/C HNCPs | CFA-coated by chitosan (CCFAICS) | Chitosan-coated CFA composite | Porous pellets | Floral-shaped nanosilica | |
| Environmental sample | Industrial effluents | Industrial wastewater | Water | Water | Water | Wastewater | CFA slurry |
| Class of CFA used | F | F | F | F | – | C | F |
| Optimum pH | 3.0 (Cr) and 5.0 (Cu) | – | – | – | 5.0 | pH 7.0 (Cr2+, Pb2+) and pH 8.0 (Zn2+) | 5.0 (Pb) and 7.0 (Zn) |
| Contaminant | Cu(II) and Cr(VI) | Hg(II) | Cd(II) | As, Cr, Zn, and Cu | Cr(VI) | Cr(III), Pb(II), and Zn(II) | Ni, Co, Al, Pb, Cr, Zn, Mn, Cd, and Cu |
| Method | Alkaline fusion | Microwave irradiation | Hydrothermal method | Coating | Coating | Pulverization (pelletizing) | Sol-gel technique |
| Order model and kinetic models | Pseudo-second-order | Pseudo-second-order | Pseudo-second order | Pseudo second order | – | Pseudo-second-order | – |
| Adsorption type | Chemisorption | Physisorption and chemisorption | – | Chemisorption | – | Chemisorption | – |
| Adsorption capacity (mg/g) | 48.31 (Cr) and 147.06 (Cu) | 0.44 | – | 19.10–55.52 | 33.27 | 0.22 Pb (II), 0.27 Zn (II) and 0.44 Cr (III) | – |
| Removal efficiency (%) | – | 94.00 | 97.41 | – | – | – | 40.00–90.00 |
| Isotherm model | Langmuir | Freundlich | Langmuir | Freundlich | Langmuir | Langmuir | – |
| BET surface area (m2/g) | 154.11 | – | 25.50 | 1.40 | 26.95 | 23.41 | – |
| Size of the material (nm) | 100.00 | – | – | 200.08 | – | 3.50–4.50 | 20.00–70.00 |
| Country | Korea | Canada | South Africa | Poland | China | Greece | India |
| References | [75] | [92] | [12] | [79] | [45] | [4] | [72] |
In Table 1, most of the researchers exploited class F CFA (Canada, China, Greece, India, Korea, Poland, and South Africa). Class F CFA was used because of its low calcium oxide content (less than 10 wt%) and it is rich in Al2O3, Fe2O3 and SiO2 (greater than 70 wt% combined) according to the American Society for Testing Materials Standards. As mentioned in the introduction section, it mostly originates from bituminous and anthracite coals and has higher LOI than class C CFA and has pozzolanic properties [27]. Class F CFA from South Africa has been confirmed to be a good substrate for silica nanoparticle and zeolite syntheses due to its compositional dominance of silicate and aluminosilicates [79,93]. Methods or techniques found to be mostly used to synthesize nanomaterials include alkaline fusion, pulverization, sol-gel technique, hydrothermal method, coating and microwave irradiation. The kinetic and isotherm models of CFA-based nanomaterials dominating others were pseudo-second order and Langmuir models, respectively. The pseudo-second order kinetic model followed in some studies assumes that the rate-limiting step is chemisorption in which adsorption increases with an increasing temperature and predicts the behaviour over the whole range of adsorption process [91,92,94]. Optimum pH and removal efficiency ranged between 3.0 and 8.0, and 40.00–97.41 %, respectively. The removed trace metals were Cu (II), Cr (VI), Hg (II), Cd (II), As, Zn, Cr (III), Pb (II), Ni, Co, and Mn. Ideal surface areas of reviewed nanomaterials (Table 1) ranged between 1.40 and 154.11 m2. g−1 which is a good range for suitable nanomaterials while adsorption capacity ranged between 0.22 and 147.06 mg. g−1. The advantages presented by the use of CFA make this industrial by-product a highly sought precursor material to synthesize other nanomaterial composites for trace metal removal from wastewaters.
3. Conclusion and future perspectives
Worldwide, high amounts of CFA are still being produced due to high usage of coal for generation of electricity. Environmental pollution due to its disposal have resulted to exploration of its potential for reuse. CFA can be recycled and applied in water treatment processes due to its noteworthy properties.. Reuse processes can contribute significantly to the reduction of this voluminous waste material and provide low-cost adsorbent materials. This review highlights the advantages of CFA as an excellent adsorbent material for removal of metals from environmental samples. Findings show that CFA as an adsorbent (nano-sized particles) or as a precursor material to synthesize nanomaterials has the potential for removal of trace metals from wastewater and can be modified to improve its adsorption capacity. Based on these findings, researchers and scientists can benefit from the reuse of CFA as it does not require an intensive pretreatment process for its application. CFA is a low-cost by-product material and is readily available in large quantities.
Data availability
Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication.
Has data associated with your study been deposited into a publicly available repository?No.
Please select why. Please note that this statement will be available alongside your article upon publication.
CRediT authorship contribution statement
Alexis Munyengabe: Writing – review & editing, Writing – original draft, Software, Data curation, Conceptualization. Maria Banda: Writing – original draft, Supervision, Funding acquisition, Conceptualization. Wilma Augustyn: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Khathutshelo Netshiongolwe: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Denga Ramutshatsha-Makhwedzha: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors of this review are thankful the Tshwane University of Technology (TUT) for financial support and infrastructure, and the National Research Foundation of South Africa (Grant no. 129752) for funding.
Contributor Information
Alexis Munyengabe, Email: munyengabea@tut.ac.za.
Maria Banda, Email: mashigomf@tut.ac.za.
Wilma Augustyn, Email: augustynw@tut.ac.za.
Khathutshelo Netshiongolwe, Email: netshiongolweke@tut.ac.za.
Denga Ramutshatsha-Makhwedzha, Email: makhwezhad@tut.ac.za.
References
- 1.Briffa J., Sinagra E., Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y., Li J., Yan K., Cao L., Cheng F. A prospective process for alumina extraction via the co-treatment of coal fly ash and bauxite red mud: investigation of the process. Hydrometallurgy. 2019;186:98–104. doi: 10.1016/j.hydromet.2019.04.011. [DOI] [Google Scholar]
- 3.Liu C., Ma S., Zheng S., Luo Y., Ding J., Wang X., Zhang Y. Combined treatment of red mud and coal fly ash by a hydro-chemical process. Hydrometallurgy. 2018;175:224–231. doi: 10.1016/j.hydromet.2017.11.005. [DOI] [Google Scholar]
- 4.Papandreou A.D., Stournaras C.J., Panias D., Paspaliaris I. Adsorption of Pb (II), Zn (II) and Cr (III) on coal fly ash porous pellets. Miner. Eng. 2011;24(13):1495–1501. doi: 10.1016/j.mineng.2011.07.016. [DOI] [Google Scholar]
- 5.saad Algarni T., Al-Mohaimeed A.M. vol. 102339. Journal of King Saud University-Science; 2022. (Water Purification by Absorption of Pigments or Pollutants via Metaloxide). [DOI] [Google Scholar]
- 6.Alsafran M., Saleem M.H., Al Jabri H., Rizwan M., Usman K. Principles and applicability of integrated remediation strategies for heavy metal Removal/Recovery from contaminated environments. J. Plant Growth Regul. 2023;42(6):3419–3440. doi: 10.1007/s00344-022-10803-1. [DOI] [Google Scholar]
- 7.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology: Volume 3: Environmental Toxicology. 2012:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandara K.R., Manage P.M. Heavy metal contamination in the Coastal environment and trace level Identification. 2022. [DOI]
- 9.Singh S., Babu K.S. Ecological Risk Assessment of heavy metal pollution in water resources. Metal Organic Frameworks for Wastewater Contaminant Removal. 2023:281–297. doi: 10.1002/9783527841523.ch12. [DOI] [Google Scholar]
- 10.Siddiqui S.I., Chaudhry S.A. Arsenic removal from water using nanocomposites: a review. Current Environmental Engineering. 2017;4(2):81–102. doi: 10.1016/j.eti.2021.102114. [DOI] [Google Scholar]
- 11.Khadir A., Mollahosseini A., Tehrani R.M., Negarestani M. Sustainable Green Chemical Processes and their Allied Applications; 2020. A Review on Pharmaceutical Removal from Aquatic Media by Adsorption: Understanding the Influential Parameters and Novel Adsorbents; pp. 207–265. [DOI] [Google Scholar]
- 12.Umejuru E.C., Prabakaran E., Pillay K. Coal fly ash coated with carbon hybrid nanocomposite for remediation of cadmium (II) and photocatalytic application of the spent adsorbent for reuse. Results in Materials. 2020;7 doi: 10.1016/j.rinma.2020.100117. [DOI] [Google Scholar]
- 13.Arzoo A. Effect of nickel on germination, seedling growth and biochemical alterations of Sesamum orientale L. Bioscience Biotechnology Research Communications. Special Issue. 2020;13(2):143–146. [Google Scholar]
- 14.Gupta V.K., Ali I. Removal of lead and chromium from wastewater using bagasse fly ash-a sugar industry waste. J. Colloid Interface Sci. 2004;271(2):321–328. doi: 10.1016/j.jcis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Qasem N.A., Mohammed R.H., Lawal D.U. Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean Water. 2021;4(1):36. doi: 10.1038/s41545-021-00127-0. [DOI] [Google Scholar]
- 16.Liu S., Costa M. Handbook on the Toxicology of Metals. Academic Press; 2022. Carcinogenicity of metal compounds; pp. 507–542. [DOI] [Google Scholar]
- 17.Wanguwa E., Asthana S. Biosorption of heavy metals from wastewater using agricultural waste biomass. Journal of Emerging Technologies and Innovative Research. 2022;9(2) [Google Scholar]
- 18.Gao Y., Zhao S., Qiao Z., Zhou Y., Song B., Wang Z., Wang J. Reverse osmosis membranes with guanidine and amine enriched surface for biofouling and organic fouling control. Desalination. 2018;430:74–85. doi: 10.1016/j.desal.2017.12.055. [DOI] [Google Scholar]
- 19.Dassanayake R.S., Acharya S., Abidi N. Recent advances in biopolymer-based dye removal technologies. Molecules. 2021;26(15):4697. doi: 10.3390/molecules26154697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munyengabe A., Zvinowanda C., Ramontja J., Zvimba J.N. Effective desalination of acid mine drainage using an advanced oxidation process: sodium ferrate (VI) salt. Water. 2021;13(19):2619. doi: 10.3390/w13192619. [DOI] [Google Scholar]
- 21.Raji Y., Nadi A., Mechnou I., Saadouni M., Cherkaoui O., Zyade S. High adsorption capacities of crystal violet dye by low-cost activated carbon prepared from Moroccan Moringa oleifera wastes: characterization, adsorption and mechanism study. Diam. Relat. Mater. 2023;135 doi: 10.1016/j.diamond.2023.109834. [DOI] [Google Scholar]
- 22.Abussaud B., Asmaly H.A., Saleh T.A., Gupta V.K., Atieh M.A. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J. Mol. Liq. 2016;213:351–359. doi: 10.1016/j.molliq.2015.08.044. [DOI] [Google Scholar]
- 23.Adam A.M., Saad H.A., Atta A.A., Alsawat M., Hegab M.S., Altalhi T.A., Refat M.S. An environmentally friendly method for removing Hg (II), Pb (II), Cd (II) and Sn (II) heavy metals from wastewater using novel metal-carbon-based composites. Crystals. 2021;11(8):882. doi: 10.3390/cryst11080882. [DOI] [Google Scholar]
- 24.Ramutshatsha-Makhwedzha D., Mbaya R., Mavhungu M.L. Application of activated carbon banana peel coated with Al2O3-chitosan for the adsorptive removal of lead and cadmium from wastewater. Materials. 2022;15(3):860. doi: 10.1016/j.heliyon.2022.e09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astm C. 618, Standard specification for coal fly ash and raw or calcined natural pozzolan for use as a mineral admixture in concrete. Annu. Book ASTM Stand. 2005;4 [Google Scholar]
- 26.Bhatt A., Priyadarshini S., Mohanakrishnan A.A., Abri A., Sattler M., Techapaphawit S. Physical, chemical, and geotechnical properties of coal fly ash: a global review. Case Stud. Constr. Mater. 2019;11 doi: 10.1016/j.cscm.2019.e00263. [DOI] [Google Scholar]
- 27.Alterary S.S., Marei N.H. Fly ash properties, characterization, and applications: a review. J. King Saud Univ. Sci. 2021;33(6) doi: 10.1016/j.jksus.2021.101536. [DOI] [Google Scholar]
- 28.Gollakota A.R., Volli V., Shu C.M. Progressive utilisation prospects of coal fly ash: a review. Sci. Total Environ. 2019;672:951–989. doi: 10.1016/j.scitotenv.2019.03.337. [DOI] [PubMed] [Google Scholar]
- 29.Saleh T.A., Mustaqeem M., Khaled M. Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ. Nanotechnol. Monit. Manag. 2022;17 doi: 10.1016/j.enmm.2021.100617. [DOI] [Google Scholar]
- 30.Kelleher B.P., O'Callaghan M.N., Leahy M.J., O'Dwyer T.F., Leahy J.J. The use of fly ash from the combustion of poultry litter for the adsorption of chromium (III) from aqueous solution. J. Chem. Technol. Biotechnol.: International Research in Process, Environmental & Clean Technology. 2002;77(11):1212–1218. doi: 10.1002/jctb.689. [DOI] [Google Scholar]
- 31.Zango Z.U., Sambudi N.S., Jumbri K., Ramli A., Abu Bakar N.H.H., Saad B., Sulieman A. An overview and evaluation of highly porous adsorbent materials for polycyclic aromatic hydrocarbons and phenols removal from wastewater. Water. 2020;12(10):2921. doi: 10.3390/w12102921. [DOI] [Google Scholar]
- 32.Afolalu S.A., Okwilagwe O., Yusuf O.O., Oloyede O.R., Banjo S.O., Ademuyiwa F. Advanced Manufacturing in Biological, Petroleum, and Nanotechnology Processing: Application Tools for Design, Operation, Cost Management, and Environmental Remediation. Springer International Publishing; Cham: 2022. Overview of nano-agro-composite additives for wastewater and effluent treatment; pp. 223–236. [DOI] [Google Scholar]
- 33.Ayeni A.O., Oladokun O., Orodu O.D. Springer Nature; 2022. Advanced Manufacturing in Biological, Petroleum, and Nanotechnology Processing: Application Tools for Design, Operation, Cost Management, and Environmental Remediation. [DOI] [Google Scholar]
- 34.Ramutshatsha-Makhwedzha D., Munyengabe A., Mavhungu M.L., Mbaya R., Baloyi J. Breakthrough studies for the sorption of methylene blue dye from wastewater samples using activated carbon derived from waste banana peels. Biomass Conversion and Biorefinery. 2023:1–13. doi: 10.1007/s13399-023-04329-z. [DOI] [Google Scholar]
- 35.Ramutshatsha-Makhwedzha D., Mbaya T., Mavhungu A., Mavhunga M.L., Mbaya R. Journal of the Iranian Chemical Society; 2023. Adsorptive Removal of Cd2+, Pb2+, and Fe2+ from Acid Mine Drainage Using a Mixture of Waste Orange and Lemon Activated Carbon (WOLAC): Equilibrium Study; pp. 1–15. [DOI] [Google Scholar]
- 36.Shahzadi I., Mubarak S., Farooq A., Hussain N. Apple peels as a potential adsorbent for removal of Cu and Cr from wastewater. AQUA-Water Infrastructure, Ecosystems and Society. 2023 doi: 10.2166/aqua.2023.216. [DOI] [Google Scholar]
- 37.Crini G., Lichtfouse E., Wilson L.D., Morin-Crini N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019;17:195–213. doi: 10.1007/s10311-018-0786-8. [DOI] [Google Scholar]
- 38.Almeida-Naranjo C.E., Guerrero V.H., Villamar-Ayala C.A. Emerging contaminants and their removal from aqueous media using conventional/non-conventional adsorbents: a glance at the relationship between materials, processes, and technologies. Water. 2023;15(8):1626. doi: 10.3390/w15081626. [DOI] [Google Scholar]
- 39.Srivastava V.C., Mall I.D., Mishra I.M. Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash. Chem. Eng. Process: Process Intensif. 2009;48(1):370–379. doi: 10.1016/j.cep.2008.05.001. [DOI] [Google Scholar]
- 40.Singh N.B., Nagpal G., Agrawal S. Water purification by using adsorbents: a review. Environ. Technol. Innovat. 2018;11:187–240. doi: 10.1016/j.eti.2018.05.006. [DOI] [Google Scholar]
- 41.Gacem M.A., Telli A., Khelil A.O.E.H. Aquananotechnology. Elsevier; 2021. Nanomaterials for detection, degradation, and adsorption of pesticides from water and wastewater; pp. 325–346. [DOI] [Google Scholar]
- 42.Gadore V., Ahmaruzzaman M. Tailored fly ash materials: a recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Proc. Eng. 2021;41 doi: 10.1016/j.jwpe.2020.101910. [DOI] [Google Scholar]
- 43.Singh A., Pal D.B., Mohammad A., Alhazmi A., Haque S., Yoon T., Gupta V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: new insight. Bioresour. Technol. 2022;343 doi: 10.1016/j.biortech.2021.126154. [DOI] [PubMed] [Google Scholar]
- 44.Nandanwar P., Jugade R., Gomase V., Shekhawat A., Bambal A., Saravanan D., Pandey S. Chitosan-Biopolymer-entrapped activated charcoal for adsorption of reactive orange dye from aqueous phase and CO2 from gaseous phase. Journal of Composites Science. 2023;7(3):103. doi: 10.3390/jcs7030103. [DOI] [Google Scholar]
- 45.Wen Y., Tang Z., Chen Y., Gu Y. Adsorption of Cr (VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chem. Eng. J. 2011;175:110–116. doi: 10.1016/j.cej.2011.09.066. [DOI] [Google Scholar]
- 46.Sahoo T.R., Prelot B. Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier; 2020. Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology; pp. 161–222. [DOI] [Google Scholar]
- 47.Du K., Qiao L. Medical Textiles from Natural Resources. Woodhead Publishing; 2022. Biotextile-based adsorbents for medical applications; pp. 117–135. [DOI] [Google Scholar]
- 48.Taghizade Firozjaee T., Mehrdadi N., Baghdadi M., Nabi Bidhendi G.R. Application of nanotechnology in pesticides removal from aqueous solutions-a review. Int. J. Nanosci. Nanotechnol. 2018;14(1):43–56. https://www.ijnnonline.net/article_30199_d07958a9d6abde6ff5221c123ae3f28e.pdf [Google Scholar]
- 49.Ahamed M.I., Lichtfouse E., Asiri A.M., editors. Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water. Springer; 2021. [DOI] [Google Scholar]
- 50.Wilkins N.S., Rajendran A., Farooq S. Dynamic column breakthrough experiments for measurement of adsorption equilibrium and kinetics. Adsorption. 2021;27(3):397–422. doi: 10.1007/s10450-020-00269-6. [DOI] [Google Scholar]
- 51.Hussain Z., Zhang H., Chang N., Wang H. Synthesis of porous materials by the modification of coal fly ash and its environmentally friendly use for the removal of heavy metals from wastewater. Front. Environ. Sci. 2022;10 doi: 10.3389/fenvs.2022.1085326. [DOI] [Google Scholar]
- 52.Aigbe U.O., Ukhurebor K.E., Onyancha R.B., Osibote O.A., Darmokoesoemo H., Kusuma H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J. Mater. Res. Technol. 2021;14:2751–2774. doi: 10.1016/j.jmrt.2021.07.140. [DOI] [Google Scholar]
- 53.Ankrah A.F., Tokay B., Snape C.E. Heavy metal removal from aqueous solutions using fly-ash derived zeolite NaP1. Int. J. Environ. Res. 2022;16(2):17. doi: 10.1007/s41742-022-00395-9. [DOI] [Google Scholar]
- 54.Buema G., Trifas L.M., Harja M. Removal of toxic copper ion from aqueous media by adsorption on fly ash-derived zeolites: kinetic and equilibrium studies. Polymers. 2021;13:3468. doi: 10.3390/polym13203468. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S., Wu H. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard Mater. 2006;136(3):482–501. doi: 10.1016/j.jhazmat.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 56.Chatterjee A., Xijun H.U., Lam F.L.Y. Modified coal fly ash waste as an efficient heterogeneous catalyst for dehydration of xylose to furfural in biphasic medium. Fuel. 2019;239:726–736. doi: 10.1016/j.fuel.2018.10.138. [DOI] [Google Scholar]
- 57.Rao M., Parwate A.V., Bhole A.G. Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manag. 2002;22(7):821–830. doi: 10.1016/S0956-053X(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 58.Buzukashvili S., Hu W., Sommerville R., Brooks O., Kökkılıç O., Rowson N.A., Waters K.E. Magnetic zeolite: synthesis and copper adsorption followed by magnetic separation from treated water. Crystals. 2023;13(9):1369. doi: 10.3390/cryst13091369. [DOI] [Google Scholar]
- 59.Ahmaruzzaman M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011;166(1–2):36–59. doi: 10.1016/j.cis.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Potgieter J.H., Pardesi C., Pearson S. A kinetic and thermodynamic investigation into the removal of methyl orange from wastewater utilizing fly ash in different process configurations. Environ. Geochem. Health. 2021;43:2539–2550. doi: 10.1007/s10653-020-00567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pholosi A., Naidoo E.B., Ofomaja A.E. Batch and continuous flow studies of Cr (VI) adsorption from synthetic and real wastewater by magnetic pine cone composite. Chem. Eng. Res. Des. 2020;153:806–818. doi: 10.1016/j.cherd.2019.11.004. [DOI] [Google Scholar]
- 62.Mathapati M., Amate K., Prasad C.D., Jayavardhana M.L., Raju T.H. A review on fly ash utilization. Mater. Today: Proc. 2022;50:1535–1540. doi: 10.1016/j.matpr.2021.09.106. [DOI] [Google Scholar]
- 63.Onutai S., Kobayashi T., Thavorniti P., Jiemsirilers S. Removal of Pb2+, Cu2+, Ni2+, Cd2+ from wastewater using fly ash based geopolymer as an adsorbent. Key Eng. Mater. 2018;773:373–378. doi: 10.4028/www.scientific.net/KEM.773.373. [DOI] [Google Scholar]
- 64.Tomasz K., Anna K., Ryszard C. Effective adsorption of lead ions using fly ash obtained in the novel circulating fluidized bed combustion technology. Microchem. J. 2019;145:1011–1025. doi: 10.1016/j.microc.2018.12.005. [DOI] [Google Scholar]
- 65.Zhao Y., Luan H., Yang B., Li Z., Song M., Li B., Tang X. Adsorption of Pb, Cu and Cd from water on coal fly ash-red mud modified composite material: characterization and mechanism. Water. 2023;15(4):767. doi: 10.3390/w15040767. [DOI] [Google Scholar]
- 66.Abu-Daabes M.A., Abu Zeitoun E., Mazi W. Competitive adsorption of quaternary metal ions, Ni2+, Mn2+, Cr6+, and Cd2+, on acid-treated activated carbon. Water. 2023;15(6):1070. [Google Scholar]
- 67.Sun S., Tang Y., Li J., Kou J., Liu Y. Fly ash derived calcium silicate hydrate as a highly efficient and fast adsorbent for Cu (ii) ions: role of copolymer functionalization. RSC Adv. 2022;12(35):22843–22852. doi: 10.1039/D2RA03007A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ajorloo M., Ghodrat M., Scott J., Strezov V. Heavy metals removal/stabilization from municipal solid waste incineration fly ash: a review and recent trends. J. Mater. Cycles Waste Manag. 2022;24(5):1693–1717. doi: 10.1007/s10163-022-01459-w. [DOI] [Google Scholar]
- 69.Kumar M., Goswami L., Singh A.K., Sikandar M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon. 2019;5(10) doi: 10.1016/j.heliyon.2019.e02562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Gisi S., Lofrano G., Grassi M., Notarnicola M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustainable Materials and Technologies. 2016;9:10–40. doi: 10.1016/j.susmat.2016.06.002. [DOI] [Google Scholar]
- 71.Gupta V.K., Sharma S. Removal of zinc from aqueous solutions using bagasse fly ash-a low-cost adsorbent. Ind. Eng. Chem. Res. 2003;42(25):6619–6624. doi: 10.1021/ie0303146. [DOI] [Google Scholar]
- 72.Umejuru E.C., Prabakaran E., Pillay K. Coal fly ash decorated with graphene and polyaniline nanocomposites for effective adsorption of hexavalent chromium and its reuse for photocatalysis. ACS Omega. 2023 doi: 10.1021/acsomega.2c05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yadav V.K., Amari A., Wanale S.G., Osman H., Fulekar M.H. Synthesis of floral-shaped nanosilica from coal fly ash and its application for the remediation of heavy metals from fly ash aqueous solutions. Sustainability. 2023;15(3):2612. doi: 10.3390/su15032612. [DOI] [Google Scholar]
- 74.Naushad M., editor. A New Generation Material Graphene: Applications in Water Technology. Springer international publishing; Cham: 2019. pp. 21–42. [DOI] [Google Scholar]
- 75.Angaru G.K.R., Choi Y.L., Lingamdinne L.P., Choi J.S., Kim D.S., Koduru J.R., Chang Y.Y. Facile synthesis of economical feasible fly ash–based zeolite–supported nano zerovalent iron and nickel bimetallic composite for the potential removal of heavy metals from industrial effluents. Chemosphere. 2021;267 doi: 10.1016/j.chemosphere.2020.128889. [DOI] [PubMed] [Google Scholar]
- 76.Akinyemi S., Gitari M., editors. Coal Fly Ash Beneficiation: Treatment of Acid Mine Drainage with Coal Fly Ash. BoD-Books on Demand; 2018. [DOI] [Google Scholar]
- 77.Asl S.M.H., Javadian H., Khavarpour M., Belviso C., Taghavi M., Maghsudi M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: a review. J. Clean. Prod. 2019;208:1131–1147. doi: 10.1016/j.jclepro.2018.10.186. [DOI] [Google Scholar]
- 78.Abbo H.S., Gupta K.C., Khaligh N.G., Titinchi S.J. Carbon nanomaterials for wastewater treatment. ChemBioEng Rev. 2021;8(5):463–489. doi: 10.1002/cben.202100003. [DOI] [Google Scholar]
- 79.Imoisili P.E., Nwanna E.C., Jen T.C. Facile preparation and characterization of silica nanoparticles from South Africa fly ash using a sol-gel hydrothermal method. Processes. 2022;10(11):2440. doi: 10.3390/pr10112440. [DOI] [Google Scholar]
- 80.Adamczuk A., Kołodyńska D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015;274:200–212. doi: 10.1016/j.cej.2015.03.088. [DOI] [Google Scholar]
- 81.Al-Zboon K., Al-Harahsheh M.S., Hani F.B. Fly ash-based geopolymer for Pb removal from aqueous solution. J. Hazard Mater. 2011;188(1–3):414–421. doi: 10.1016/j.jhazmat.2011.01.133. [DOI] [PubMed] [Google Scholar]
- 82.Huang X., Zhao H., Hu X., Liu F., Wang L., Zhao X., Ji P. Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+ J. Hazard Mater. 2020;392 doi: 10.1016/j.jhazmat.2020.122461. [DOI] [PubMed] [Google Scholar]
- 83.Koshlak H. Synthesis of zeolites from coal fly ash using alkaline fusion and its applications in removing heavy metals. Materials. 2023;16(13):4837. doi: 10.3390/ma16134837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Guo Y., Yang Z., Cai H., Xavier Q. Synthesis of zeolites using fly ash and their application in removing heavy metals from waters. Sci. China Earth Sci. 2003;46:967–976. doi: 10.1360/02yd0487. [DOI] [Google Scholar]
- 85.Tauanov Z., Tsakiridis P.E., Mikhalovsky S.V., Inglezakis V.J. Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury (II) removal from water. J. Environ. Manag. 2018;224:164–171. doi: 10.1016/j.jenvman.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 86.Mofulatsi M.W., Prabakaran E., Velempini T., Green E., Pillay K. Preparation of manganese oxide coated coal fly ash adsorbent for the removal of lead and reuse for latent fingerprint detection. Microporous Mesoporous Mater. 2022;329 doi: 10.1016/j.micromeso.2021.111480. [DOI] [Google Scholar]
- 87.Asl S.M.H., Ghadi A., Baei M.S., Javadian H., Maghsudi M., Kazemian H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: a review. Fuel. 2018;217:320–342. doi: 10.1016/j.fuel.2017.12.111. [DOI] [Google Scholar]
- 88.Kim H.J., Joshi M.K., Pant H.R., Kim J.H., Lee E., Kim C.S. One-pot hydrothermal synthesis of multifunctional Ag/ZnO/fly ash nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2015;469:256–262. doi: 10.1016/j.colsurfa.2015.01.032. [DOI] [Google Scholar]
- 89.Mashile G.P., Mpupa A., Nqombolo A., Dimpe K.M., Nomngongo P.N. Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J. Water Proc. Eng. 2020;33 doi: 10.1016/j.jwpe.2019.101011. [DOI] [Google Scholar]
- 90.Ribeiro J., DaBoit K., Flores D., Kronbauer M.A., Silva L.F. Extensive FE-SEM/EDS, HR-TEM/EDS and ToF-SIMS studies of micron-to nano-particles in anthracite fly ash. Sci. Total Environ. 2013;452:98–107. doi: 10.1016/j.scitotenv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 91.Etale A., Tavengwa N.T., Pakade V.E. Metal adsorption by coal fly ash: the role of nano-sized materials. Coal Fly Ash Benef.-Treat. Acid Mine Drain. with Coal Fly Ash. 2018 doi: 10.5772/intechopen.69426. [DOI] [Google Scholar]
- 92.Attari M., Bukhari S.S., Kazemian H., Rohani S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J. Environ. Chem. Eng. 2017;5(1):391–399. doi: 10.1016/j.jece.2016.12.014. [DOI] [Google Scholar]
- 93.Musyoka N.M. Masters Dissertation. University of Witwatersrand; 2009. Hydrothermal synthesis and optimisation of zeolite Na-Pl from South African coal fly ash. [Google Scholar]
- 94.Shukla P., Manivannan S., Mandal D. Silver zeolite in ultrasonically welded packed beds for enhanced elemental mercury capture from contaminated air stream: adsorption and kinetics study. Microporous Mesoporous Mater. 2023;359 doi: 10.1016/j.micromeso.2023.112651. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication.
Has data associated with your study been deposited into a publicly available repository?No.
Please select why. Please note that this statement will be available alongside your article upon publication.