Abstract
The study investigated the effectiveness of Mg/Al LDH-zeolite (MALZ) in immobilizing exchangeable Cr (e-Cr) within the soil. The research systematically evaluated various variables affecting the immobilization of e-Cr in contaminated soil (CS), including soil pH levels (ranging from 5.0 to 9.0), different weight ratios of MALZ (1 %, 3 %, and 5 %), durations of differing incubation periods (15, 30 and 45 days), and different SM content levels (30 %, 50 %, and 70 %). The initial concentration of Cr in the CS was maintained at 50 mg/kg. The investigation findings revealed that the optimal conditions for immobilizing the e-Cr were a soil pH of 5.0, an MALZ weight ratio of 3 %, an incubation period of 30 days, and an SM level of 70 %. Under these ideal conditions, the percentage of e-Cr within the CS decreased significantly, from 87.49 % (45.64 mg/kg) in the control treatment (CT) to just 19.82 % (10.08 mg/kg) when incubated with MALZ. The primary mechanisms responsible for immobilizing the e-Cr onto MALZ included pore filling, reduction processes, co-precipitation, organic interactions and electrostatic attractions leading to the formation of carbonate-bound complexes such as Cr(VI)-carbonate, Cr(III)-carbonate, and organic complexes. Surface functional groups on MALZ, housing iron and aluminium oxyhydroxides and silicon and oxygen elements, expedited these procedures. This study provided a valuable understanding of the mitigation of soils contaminated with chromium and contributed to understanding the relations between MALZ and the e-Cr in the soil. The discoveries carry substantial consequences for the advancement of efficient remediation technologies.
Keywords: Mg/al-LDH-Zeolite, Chromium, Contaminated soil, Immobilization, Zeolite
1. Introduction
The pollution of soil with chromium (Cr) is a matter of serious issue due to its detrimental consequences for plants, the soil-vegetable ecosystem and human health, as recorded by Nagajyoti et al. (2010) [1] and Jaishankar et al. (2014) [2]. Anthropogenic sources, such as industrial processes, sludge applications, solid waste disposal, vehicular exhaust and wastewater irrigation, have been identified as primary contributors to Cr contamination in soil, as documented by Khan et al. (2008) [3]. These human activities cause the significant discharge of heavy metals (HMs), including Cr, into the soil, leading to contamination. Wastewater irrigation, in particular, is a noteworthy source of heavy metal pollution in soils. Additionally, industrial activities and vehicle emissions are recognized as culprits in releasing Cr into the environment, where it accumulates in the soil. The leaching of Cr(VI) from stainless steel manufacturing processes significantly plays a part in the contamination of Cr(VI) in water and soils, as noted by Radhan et al. (2017) [4]. The mobility of Cr(VI) in the soil, allowing it to disperse easily through water, leads to extensive pollution over large areas, as highlighted by Li et al. (2020) [5].
The contamination of Cr in soils has been shown to significantly impact the composition of bacterial communities. Zhu et al. (2023) [6] demonstrated that chromium contamination induces alterations in bacterial communities inhabiting the CS. Specifically, the presence of Cr can impact the richness and variety of bacterial taxa, including Firmicutes and Acidobacteria. These shifts in bacterial communities have implications for the overall health of the soil and the functioning of ecosystems. Consequently, soil pollution can harm the soil-vegetable system, disrupting vegetable yield and quality, which can affect human health.
Various techniques have been utilized to treat chromium in contaminated soil (CS), with a specific approach depending on the circumstances and desired outcomes. One commonly used process involves using chemical agents to reduce and immobilize the Cr in the soil. Geelhoed et al. (2003) [7] reported that the addition of ferrous sulfate (FeSO4) effectively converts Cr(VI) to Cr(III) and precipitates it as iron/chromium hydroxide. Hu et al. (2023) [8] demonstrated the feasibility of this method through injection tests of FeSO4 solution on clayey soil contaminated with Cr(VI) under different injection pressures. In addition to ferrous sulfate, other chemicals such as oxalic acid, citric acid and hydrochloric acid have been utilized in column-leaching experiments to convert Cr from CS, as described by Sun et al. (2019) [9]. Moreover, electrokinetic remediation has emerged as a promising technique for removing Cr from the CS. Yan et al. (2018) [10] reported the successful remediation of chromium-contaminated soil using an electric field to mobilize and extract contaminants, employing iron-loaded activated carbon electrodes in a three-dimensional system. Bioremediation approaches have also been explored for Cr-contaminated soil, with certain bacteria, such as Bacillus cereus, identified for their ability to reduce and detoxify Cr Li et al. (2020) [11]. Phytoremediation, which involves using plants to remove contaminants from the soil, has been studied for Cr(VI) contamination. Su et al. (2005) [12] revealed the potential of Chinese brake fern (Pteris vittata) as a hyperaccumulator of Cr(VI) in soils. The mixed lead and chromium pollution in soil has been investigated, and treatment techniques for heavy metal-contaminated soil have been proposed by Yang et al. (2020) [13]. These studies collectively demonstrate the diverse range of methods available for treating Cr-contaminated soil, encompassing chemical, electrokinetic, bioremediation, and phytoremediation approaches. The findings underscore the importance of considering site-specific factors and the efficacy of each method in achieving the desired remediation outcomes. The study aids in developing comprehensive strategies for addressing chromium contamination in soil, with potential implications for environmental remediation and sustainable land use.
The adsorption method is a frequently employed technique for immobilizing chromium (Cr) in the CS, as outlined by Fu and Wang (2011) [14]. This method focuses on binding the Cr ion to the surface of adsorbent material, leading to their efficient removal from the soil. Increasing research has been performed to assess the efficacy of diverse adsorbents in eliminating Cr from the CS. Bagherzadeh et al. (2023) [15] reported that a nanocomposite demonstrated a remarkable capacity for removing Cr(VI) under optimal conditions, highlighting the effectiveness of activated carbon-based adsorbents in treating soil contaminated with chromium. In a separate study, Liu et al. (2020) [16] devised a modified bipolar membrane electrodialysis system to extract simultaneously trivalent chromium (Cr(III)) and hexavalent chromium (Cr(VI)) from the CS. Furthermore, Mulyanto (2023) [17] explored using Terra Rossa soil as a sorbent for eliminating hexavalent chromium from water.
In the realm of chromium (Cr) remediation in soil, Layered Double Hydroxides (LDHs) have emerged as innovative remedies. LDHs demonstrate promising capabilities in stabilizing HMs, including chromium, thereby enhancing soil fertility and water quality. The amalgamation of LDHs with electrokinetic processes has shown potential for remediating the CS, presenting a viable approach for addressing sites contaminated with Cr [18]. The utilization of LDHs in conjunction with other remediation techniques has shown considerable promise. For instance, the integration of LDHs with zero-valent iron (nZVI) has been explored for in-situ remediation of hexavalent chromium-contaminated soil [19]. Research indicates that the utilization of LDH-stabilized nZVI composited with biochar significantly improves the remediation rate of Cr-contaminated soil, leading to notable reductions in the leachability of Cr(VI) and Cr. This underscores the potential of synergizing LDHs with other remediation agents to enhance the efficiency of chromium remediation in soil. Recent studies have highlighted the potential of LDHs in soil remediation of Cr. LDHs have effectively stabilized HMs, including chromium, in soil, thereby enhancing soil fertility and water quality [8]. The combination of LDHs with electrokinetic processes has shown promise in the remediation of the CS, indicating a viable approach for addressing Cr-contaminated sites. Furthermore, LDHs intercalated with MoS42− have demonstrated targeted and effective elimination of harmful oxoanions of As(III), As(V), and Cr(VI) due to interactions between sulfur and chromium, as well as the conversion of Cr(VI) to Cr(III) [20].
While Mg/Al-LDH-Zeolite (MALZ) has been extensively employed to treat water and wastewater contaminants, its application for immobilizing HMs in soil has been relatively limited. In a related report, Ma et al. (2016) [20] explored the selective and efficient removal of HMs, including copper (Cu2+), lead (Pb2+), silver (Ag+), and mercury (Hg+), using LDH intercalated with the MoS42− ion. Guaya et al. (2022) [21] explored the removal of phosphate from wastewater using Mg–Al-LDH doped Mn2+/Zn2+/Fe3+ oxy(hydroxide) nanoparticles. Zhang et al. (2018) [22] discussed the improvement in hexavalent chromium (Cr(VI)) removal using core-shell zeolites/LDHs prepared from metallic compounds of diverse varieties within constructed rapid infiltration systems. This research focused on the application of hydrotalcite, a type of LDH, in the immobilization of iodate (IO3−) in soil. Moreover, Shi et al. (2009) [23] provide an overview of the use of natural zeolite, including its effectiveness as an alteration for heavy metal-polluted soils.
The utilization of MALZ for chromium (Cr) immobilization in the CS represents a recent and noteworthy advancement that has garnered considerable attention from the environmental research community and practitioners. This innovative approach holds promise for effectively sequestering Cr in the CS, signalling a significant development in material research and efficacy enhancement for soil remediation. The emergence of MALZ as a promising material for addressing Cr contamination in soil not only paves the way for further exploration and application in this specific context but also lays the groundwork for potential studies and applications in remediation efforts targeting soil contaminated with other HMs and harmful nanoparticles. These findings mark a pivotal advancement in understanding the potential of advanced materials in addressing increasingly complex environmental challenges.
The main aim of this study is to explore the utilization of MALZ as a sorbent material for immobilizing chromium (Cr) in CS. The research will specifically evaluate the impact of different environmental variables on the efficacy of Cr immobilization in the CS. These factors encompass the impact of the ratio of MALZ on the weight of CS, soil pH, incubation time, and soil moiture (SM) content.
2. Material and methods
2.1. Chemicals
Chemicals of Al(NO3)3, Mg(NO3)2, K2Cr2O7, NaOH, H2SO4, (Fe(NH4)2(SO4)2.6H2O) and Na2CO3 were acquired from Merck, Germany. The zeolite used in the experiments was obtained from Nito Funka Kogyo K. K. Company, Japan.
2.2. Preparation of MALZ
The synthesis of MALZ was carried out employing the co-precipitation technique, as previously described in our prior publication [24]. The natural zeolite employed in the experiments exhibited a small particle size of <1 nm. The detailed steps of the composite process are presented as follows:
Step 1: Synthesis of the Mg/Al LDH solution.
The initial step in the synthesis process involves preparing a 100 mL solution comprising 0.01 mol of Al(NO3)3 and 0.02 mol of Mg(NO3)2. This solution acts as the foundational precursor for forming LDHs, where Mg and Al ions combine to create a layered double hydroxide structure capable of efficient anion exchange processes.
Step 2: Incorporation of zeolite.
Subsequently, a specific amount of zeolite is introduced into the solution to achieve a desired ratio of 30 % Mg/Al LDH to 70 % zeolite. The selected zeolite, characterized by particle sizes <1 mm, augments the composite material's surface area, thereby facilitating enhanced ion exchange and adsorption phenomena.
Step 3: pH adjustment and agitation.
To further the synthesis process, a solution containing 1 M NaOH and 0.5 M Na2CO3 is gradually introduced into the mixture until a pH level of 11 is attained. The pH adjustment plays a pivotal role in optimizing the precipitation kinetics of LDHs and promoting the development of the MALZ composite structure. The mixture is continuously stirred for 4 h at 80 °C, ensuring homogeneous precipitation of LDHs and their uniform dispersion within the zeolite matrix.
Step 4: Separation, purification, and drying.
Following the stirring process, the mixture undergoes centrifugation to segregate the solid phase from the liquid phase, comprising the MALZ composite. The solid material is subjected to five wash cycles using double-distilled water to eliminate residual soluble impurities. Finally, the purified solid is dried at 100 °C for 24 h. This drying step removes excess moisture and enhances the composite material's structural integrity, ensuring its stability and functionality in subsequent applications. It was then dried at 100 °C for 24 h, resulting in the formation of the MALZ.
2.3. Experimental setup
Soil preparation for experiments: A pristine soil sample was obtained from uncontaminated hill soil covered with shrub vegetation at Thai Nguyen University of Agriculture and Forestry, Thai Nguyen City, Vietnam. The soil was dried upon collection, and any root debris and residual plant material were meticulously removed. Subsequently, the wholly dried soil was finely ground, sieved through a 0.5 mm sieve, and then stored in nylon bags to preserve the soil samples. The soil underwent anion analysis (CrO42−), physical composition assessment, and soil pH determination. To introduce artificial anions (CrO42−) into the soil, K2Cr2O7 with a concentration of 50 mg Cr/kg soil was employed. The experiments were carried out using 50 g of soil enclosed in plastic bags and incubated at room temperature (26 ± 2 °C). Each experimental setup was replicated three times. The specific experiments conducted are delineated below:
Experiment 1: Impact of the ratio of MALZ and soil on Cr immobilization: Soil samples, including both CrO42− introduced samples and control samples contaminated with the Cr at a concentration of 50 mg/kg, were blended with varying proportions (1–5% w/w) of MALZ. The mixtures were then subjected to a 30-day incubation period under conditions maintaining 70 % moisture at the predetermined optimal pH. Anion (CrO42−) analyses were conducted before and after the adsorption process. Experiment 2: Impact of soil pH on Cr immobilization: Following the introduction of anion (CrO42−), the soil samples were adjusted to pH levels of 5, 6, 7, 8 and 9. Subsequently, the soil was mixed with the predetermined quantity of MALZ, as determined in Experiment 1. Incubation was carried out for 30 days while maintaining 70 % moisture. Experiment 3: Impact of moisture content on Cr immobilization: Each soil sample containing the introduced anion (CrO42−) was combined with the predetermined quantity of MALZ at the established soil pH from Experiment 2. Incubation was conducted at 30 %–70 % moisture levels for 30 days. Experiment 4: Influence of incubation time on Cr immobilization: Soil samples containing introduced anion (CrO42−) were combined with the predetermined quantity of MALZ at established soil pH conditions and moisture content from Experiments 1, 2, and 3. Incubation durations of 15, 30, and 45 days were employed, maintaining 70 % moisture throughout the experiments.
2.4. Analytical methods
Material properties: The pore volume and surface area of MALZ were analyzed employing the Brunauer-Emmet-Teller (BET) technique utilizing an SA 3000 instrument (Coulter, USA). Additionally, the surface morphology of the MALZ was investigated applying a scanning electron microscope (SEM) combined with Energy Dispersive X-ray (EDX) spectroscopy at an acceleration voltage of 2.0 kV. This investigation involved employing various magnifications following the milling process.
Method for the Cr in soil: The procedure for analyzing the form of chromium (Cr) in soil involves a sequential leaching process using the Tessier extraction method, as described by Dang et al. (2021) [24]. This method aims to quantify the content of individual metals in each leachate phase, corresponding to their various forms in the soil. The procedure consists of five main steps, each targeting a specific form of metal in the soil: Exchangeable (F1), Carbonate-bound (F2), Fe–Mn oxide-bound (F3), Organic-bound (F4), and Residual fraction (F5). In the first step, 2.0 g of the CS was weighed into a glass, and 20 mL of 1 M NH4OAc solution pre-adjusted to pH 7.0 was added (solution no. 1). The container was left to stand for 2 h at ambient temperature. After this period, the solution was filtered for the Cr analysis, and the remaining soil sample was retained to dry and kept in the same container. The second step involves adding 20 mL of 1 M NH4OAc solution pre-adjusted to the pH 5.0 (solution no. 2) to the remaining soil sample from the first step and allowing it to stand for 2 h at ambient temperature. Subsequently, the solution was filtered for heavy metal analysis, and the remaining soil sample was retained to dry and kept in the same container. In the third step, 20 mL of 0.04 M NH2OH.HCl solution prepared in 25 % HOAc (solution no. 3) was added to the remaining soil sample from the second step and kept in a water bath at 60 °C for 6 h. Following this interval, the solution was filtered for heavy metal analysis, and the remaining soil sample was retained to dry and kept in the same container. The fourth step involves adding 15 mL of 30 % H2O2 solution adjusted to pH 2.0 (solution no. 4) to the remaining soil sample from the third step and keeping it in a water bath at 80 °C for 5.5 h. After this time, the sample was cooled, and the solution was filtered for heavy metal analysis while the remaining soil sample was kept dry and in the same container. In the final step, 5 mL of 3.2 M NH4OAc solution prepared in 20 % HNO3 (solution no. 5) was included in the remaining soil sample from the fourth step. The sample is then placed on a shaker and agitated for 0.5 h before diluting with 20 mL of distilled water. After the specified time, the sample was removed, and the solution was filtered for heavy metal analysis. This sequential leaching process yields five fractions corresponding to the five main forms of metals in the soil: F1, F2, F3, F4, and F5. The total metal content in each leachate phase) was assessed utilizing an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Horiba, Japan).
pH value of soil and electrical conductivity (EC) were evaluated following the protocol outlined by Bian et al. (2013) [25]. The organic carbon (OC) assessment in soil and MALZ utilized the Walkley–Black titration method. This method involves the oxidation of organic carbon by a K2Cr2O7–H2SO4 mixture, proceeded by back titration to measure the excess dichromate content using (Fe(NH4)2(SO4)2.6H2O). Furthermore, soil texture was determined utilizing the pipetting procedure to determine the proportions of the sand, silt, and clay concentration.
Analysis of Cr(VI) and Cr(II) in soil sample: The solution quantified the total chromium using the ICP-OES. The assessment of Cr(VI) levels in the F1 solution was carried out through a colourimetric method involving the reaction with diphenylcarbazide in an acidic medium. Specifically, 50 μL of diphenylcarbazide was combined with 5 mL of the sample, followed by 100 μL of H2SO4 solution to achieve a pH range of 2.0 ± 0.5. The resultant mixture was allowed to incubate for 5–10 min to facilitate the complete development of the red-violet coloration. Subsequently, a suitable volume of the solution was transferred to a cuvette for analysis. The absorbance was measured at 540 nm using a spectrophotometer (Agilent Technologies Cary 8454 UV–vis). The concentration of Cr(VI) present in the samples was determined by referencing a calibration curve established from the analysis of a series of Cr(VI) standards prepared using the same derivatization protocol. The amount of Cr(III) was determined as the remaining residual after deducting the determined Cr(VI) concentration from the total chromium content.
3. Results and discussion
3.1. Physicochemical properties of soil and absorbent materials
In the previous investigation by Dang et al. (2021) [24], the unique characteristics of the experimental soil were evaluated. The analysis revealed deficient concentrations of certain elements, including Fe, Cd, Pb, Ca, Mn and Cr at 4.35 mg/kg, 0.50 mg/kg, 1.92 mg/kg, 2.72 mg/kg, 1.26 mg/kg, and 0.42 mg/kg, respectively. While the organic carbon (OC) content was determined as 2.03 %, the pH value of soil and electrical conductivity (EC) were verified as 27.2 μS/cm and 4.71, respectively (Table S1). These findings affirm that the soil sample employed in this study was uncontaminated with slight acidity.
Zeolite displayed specific physicochemical characteristics, featuring a pH level of 8.2 ± 0.3, an electrical conductivity (EC) measurement of 180 ± 0.05 μS/cm, and the amount of Cd and Pb at 0.052 mg/kg and 0.42 mg/kg, respectively (as outlined in Table S1). Furthermore, the physicochemical characteristics of MALZ exhibited similar patterns to the pristine zeolite counterpart. The pH value of 8.13 closely resembled Zeolite's, showcasing a mildly alkaline nature. Likewise, the EC measurement, which stood at 172 μS/cm, closely approximated the values recorded for zeolite, indicating a similarity in solute concentration. Regarding the content of HMs, a slight increase in Cd (0.056 mg/kg) was observed, while the Pb levels remained consistent at 0.44 mg/kg when compared to zeolite. This observation suggests a comparable capacity for metal adsorption between the two materials. It's worth noting that neither zeolite nor MALZ showed detectable levels of the Cr.
The elemental compositions of zeolite and MALZ were analyzed, revealing notable differences between zeolite and the as-prepared MALZ (Fig. 1a1 and b1). The major constituents found within zeolites include C, O, Na, Al, Si, K, Ca, and Fe. These elements are present in zeolites in varying proportions, with carbon accounting for 18.18 %, oxygen for 56.85 %, sodium for 1.25 %, aluminium for 3.91 %, silicon for 17.51 %, potassium for 0.62 %, calcium for 0.86 %, and iron for 0.83 % of the total composition. The MALZ is distinctly characterized in the EDS spectra data (Fig. 1b1) compared to zeolite (Fig. 1a1). In MALZ, Al increases (6.26 %), and new elements like Mg (10.09 %) emerge, while the C, Na, Si, Ca, and Fe decrease. This change in elemental composition is attributed to the effective establishment of the composite material, indicating the distinct characterization of MALZ compared to zeolite.
Fig. 1.
EDS and SEM of zeolite (a1, and a2) and MALZ (b1 and b2).
Next, Fig. 1a2 and b2 show SEM images of zeolite and MALZ. While zeolite exhibits a rod-like structure (Fig. 1a1), MALZ showed a heterogeneous structure attributed to the inclusion of Mg and Al throughout the synthesis procedure (Fig. 1b2). This heterogeneity confirmed that the composite material was successfully formed. Moreover, the BET surface area of zeolite was measured as 26.15 m2/g, which was then substantially increased to 252.66 m2/g for MALZ, indicating an enhancement in surface area due to the composite formation (Fig. 2a). In contrast, the pore volume of MALZ (0.10 cm3/g) was smaller than zeolite (0.25 cm3/g). Furthermore, the pore dimensions of MALZ and zeolite are 12.31 nm and 38.36 nm, respectively.
Fig. 2.
Adsorption-desorption isotherm for BET analysis (a) and FTIR profiles (b) of zeolite and MALZ.
The surficial functional groups of zeolite and MALZ were analyzed (Fig. 2b). Notably, both materials show the presence of –CH groups in a 600-900 cm−1 range. In the case of MALZ, a new peak appeared at 553 cm−1, ascribed to the Al–O stretching mode. The C–O functional groups positioned at 1020 and 1215 cm−1 can be noticed in both materials. In addition, while a noticeable peak at 1369 cm−1 in MALZ could be ascribed to the interlayer carbonate group, another peak detected at 1020 cm−1 could correspond to the bending modes of the Si–Al framework. Both zeolite and MALZ show a C C group at 1640 cm−1. The spectrum of MALZ also indicates the existence of hydroxyl groups at the 3460 cm−1 peak. These findings provide comprehensive insights into the structural and chemical differences between zeolite and MALZ, highlighting the impact of composite formation on their properties.
3.2. The impact of soil pH on Cr immobilization
Soil pH is a critical factor influencing HMs' chemical speciation and bioavailability in the CS [26]. Fig. 3 illustrates the effects of the soil pH on Cr immobilization in CS following the 30-day adsorption period, using a 3 % weight ratio of MALZ.
Fig. 3.
Impact of soil pH values on the transformation of Chromium forms in the CS: A 30-day adsorption, the SM of 70 % and an initial total Chromium content of 50 mg/kg, 3 % of MALZ.
Based on the study's data, the observed significant reduction in e-Cr content after a 30-day incubation with MALZ at a 3 % weight ratio across the pH spectrum from 5 to 9 underscores the efficacy of MALZ in mitigating the bioavailability of Cr in the CS. This reduction in e-Cr content aligns with utilizing MALZ as a remediation agent for Cr-contaminated soil. The ability of MALZ to effectively decrease the e-Cr content, particularly at soil pH levels of 5.0, suggests its potential for immobilizing Cr and reducing its availability for uptake by plants or leaching into groundwater. However, the data also revealed that as pH levels increased from 6.0 to 9.0, the adsorption ability of MALZ declined. This decline in adsorption capability at elevated pH levels may have implications for the overall effectiveness of MALZ in immobilizing Cr in soil with an alkaline pH. Expressly, the lowest proportion of remaining e-Cr was noted at 19.96 % (10.08 mg/kg) at a soil pH of 5.0, followed by 76.34 % (30.81 mg/kg) and 84.33 % (42.04 mg/kg) (p < 0.05) at soil pH levels of 6.0 and 7.0, respectively. The decrease in the residual e-Cr manifests similarly in soil environments with pH levels of 8 and 9. The observed decrease in the e-Cr content and the concurrent enhance in forms associated with Fe–Mn oxide occlusion (F2), carbonate binding (F3), and organic complexation (F4) following the 30-day incubation with MALZ aligns with findings from previous studies. The transformation of nearly all Cr content into the organically complexed (F4) form during the incubation period is consistent with the role of organic components in releasing metals into solution, making them readily available to plants [27]. The study supports the understanding that soil pH plays a crucial role in the speciation and stability of HMs linked with carbonate, influencing their bioavailability [28]. The findings also resonate with the research on the immobilization of the mobile cadmium and lead in the CS utilizing MALZ as an proficient sorbent, indicating the potential of this amendment for lowering the availability of HMs [24].
The study aligns with the findings that the reaction rate with nanoscale zerovalent iron (nZVI) is significantly faster, indicating efficient immobilization of Cr(VI) [29]. The distribution of species and factors influencing HMs in rhizosphere soil have been shown to impact the immobilization of the e-Cr, with the concentrations of carbonate-bound and iron manganese oxide-bound decreasing [30]. The findings of this study align with previous research examining the impact of zeolite on the immobilization of HMs in soil contaminated with municipal solid waste compost, underscoring the efficacy of zeolite as a viable option for mitigating the bioavailability of HMs in soil [31]. The transformation of heavy metal forms in soil, particularly the increase in forms associated with Fe–Mn oxide occlusion and organic complexation, has been a subject of interest in soil contamination studies due to the environmental mobility of the bioavailable heavy metal fraction [32]. Moreover, the role of natural zeolite in immobilizing HMs in the soil has been extensively studied, emphasizing its potential for lowering the bioavailability of HMs [33].
The rationale for these observations can be elucidated by the alterations in the structure and properties of MALZ as the pH increases, resulting in a decline in its adsorption capacity for Cr(VI). The MALZ, an alkaline adsorbent material, changes its surface chemistry and properties as the pH of the environment rises. This leads to the dissociation of alkaline groups on the material's surface, thereby influencing its ability to adsorb Cr(VI) ions. Specifically, as the pH increases, the hydroxide (OH−) groups on the MALZ dissociate into water (H2O) and hydroxide (OH-) ions, consequently diminishing their capacity to bond with Cr(VI) ions. Moreover, variations in pH also impact the surface charge of the adsorbent material, with an increase in pH resulting in a reduction of the material's surface charge and subsequently diminishing its adsorption capacity. This phenomenon has been substantiated by the findings of Zanin's research group [34]. Another critical aspect highlighted in the study is the transformation of Cr into other forms. Post incubation with MALZ, and there was a noticeable shift towards forms of Cr that are less exchangeable and more stable, such as those associated with Fe–Mn oxide occlusion, carbonate binding and organic complexation. This transformation process is more efficient at the neutral pH than in acidic conditions, contributing to the more significant reduction in the e-Cr.
The results prove that almost e-Cr ions were immobilized in carbonate-bound forms and organically complexed when MALZ was introduced into the CS. This underscores the effectiveness of MALZ in immobilizing the e-Cr ions in the CS, possibly attributed to the enhanced electrostatic attraction exhibited by MALZ after its modification from zeolite. The study's findings align with previous research, indicating the complexity and intricacy of soil chemistry and its influence on heavy metal speciation and mobility. This result is correlated with the fact that the diminution of Cr(VI) followed the first-order kinetics at a significant level for the examined soils, and the reduction efficiency certainly depends on the external factors, including organic matter content, the initial added chromium concentration and pH condition of soil [35]. The increase in the e-Cr form at low pH of acidic medium may be ascribed to the existence of ferric ion that enhances the reduction rate of Cr(VI), even at a trace amount of added Fe(III) in the system [36].
3.3. The impact of adsorbent ratio on Cr immobilization
The impact of varying mixture weight ratios of MALZ on the immobilization of Cr in the CS was meticulously explored in the study. The investigations were conducted across a spectrum of mixture ratios, specifically the weight of 1 %, 3 %, and 5 %, under controlled conditions of 70 % SM, a neutral soil pH of 5.0 for an adsorption duration of 30 days with a total Cr content of 50 mg/kg. The outcomes of these experiments are detailed in Fig. 4.
Fig. 4.
Influence of adsorbent (MALZ) ratio (w/w) on the transformation of Chromium forms in the CS: An incubation time of 30 days, soil pH of 5.0, SM of 70 % and an initial total Chromium content of 50 mg/kg.
The examination of the data depicted in Fig. 4 implies a significant decrease in the e-Cr content (F1) following the 30-day incubation across the range of MALZ weight ratios, between 1 % and 5 %, compared to the CT. Notably, the most pronounced decline in the e-Cr content was observed at the 3 % ratio. Beyond this ratio, increasing the ratio to 5 % resulted in only a marginal decrease in the e-Cr content. This observation aligns with adsorption and ion exchange principles, which are critical mechanisms involved in immobilizing contaminants such as chromium in soil environments. The substantial decrease in the e-Cr content might be ascribed to the adsorption capacity of the MALZ, which is known to effectively bind and immobilize Cr ions through surface complexation and ion exchange processes.
The results indicated a significant decline in e-Cr content and percentage as the MALZ weight ratio increased. Specifically, the e-Cr content decreased from 41.53 mg/kg in the CT to 7.87 mg/kg, 4.33 mg/kg, and 3.83 mg/kg for the MALZ ratios of 1 %, 3 %, and 5 %, respectively. Correspondingly, the percentage of e-Cr decreased from 80.36 % in the CT to 14.92 %, 8.27 %, and 7.48 % for the weight above ratios. Importantly, these changes were statistically significant (p ≤ 0.05), indicating a clear impact of the MALZ weight ratio on the Cr immobilization. The study's results emphasize the significant effectiveness of immobilizing Cr in the soil, particularly when using the immobilizing agent's 3 % and 5 % weight ratios. An intriguing observation from the study was the negligible difference in the effectiveness of Cr immobilization between these two ratios. This implies that the 3 % and 5 % weight ratios effectively reduced the amount and proportion of e-Cr in the soil. It indicates that increasing the ratio from 3 % to 5 % does not substantially enhance the Cr immobilization capacity. This finding is important because it suggests that the 3 % ratio was sufficiently effective, potentially offering a more cost-effective and resource-efficient approach for reducing the CS bioavailability. The phenomenon observed in the study can be elucidated by understanding that adsorbents reach a saturation point beyond which their ability to bind or immobilize additional contaminants, such as chromium (Cr), does not increase. This saturation point is a threshold where the maximum capacity of the adsorbent is achieved. The findings indicate that this threshold might be attained or nearly reached when a 3 % adsorbent ratio is used. This implies that further increasing the ratio to 5 % does not proportionally enhance the Cr immobilization. In the context of adsorption dynamics, there's a point where a balance is established between the adsorbate (Cr) and the adsorbent. Once a certain amount of adsorbent is added, the system may reach a state of dynamic equilibrium. At this juncture, the rate at which the Cr is adsorbed onto the adsorbent becomes equal to the rate at which it desorbs back into the environment, leading to no significant overall increase in immobilization efficiency. Moreover, at higher weight ratios, a phenomenon known as particle aggregation can occur, wherein adsorbent particles clump together. This aggregation diminishes the effective surface area of the adsorbent particles available for interaction with Cr ions and limits their accessibility. Such aggregation thus constrains the adsorption process's effectiveness, indicating that adding more adsorbent may not yield additional benefits in immobilizing Cr beyond a certain point.
This underscores the potential of MALZ as an effective agent for immobilizing Cr in the CT. Consistent findings have been observed in prior studies. In a study paralleling the observed reduction trends in exchangeable soil forms, Zuo et al. (2016) [37] reported similar findings using natural polymers. Their research demonstrated a decrease in the exchangeable forms of lead and cadmium correlating with an increased mass ratio of adsorbents. The current investigation results concord with prior research conducted by Igalavithana et al. (2019) [38]. This earlier study utilized pine cones and vegetable waste to prepare biochars at 2.5 % and 5.0 % rates for immobilizing efficiently exchangeable Pb in the CT. These findings collectively reinforce the efficacy of using various adsorbents, including natural polymers and biochars, to reduce the mobility of HMs in the CT.
The study observed a significant transformation of e-Cr into more stable forms post-incubation, with the predominant forms being Carbonate-bound (F3) and Organically complexed (F4). Moreover, the remaining fractions were predominantly related to Fe- and Mn-oxide occlusion (F2) and the residual form (F5). These findings underscore the potential of MALZ in altering the speciation of Cr, thereby reducing its bioavailability and environmental impact. The transformation of e-Cr into more stable forms represents a crucial step in mitigating the risks associated with Cr contamination in the soil. Furthermore, identifying specific stable forms, such as Carbonate-bound and Organically complexed fractions, provides valuable insights into the sustained stability and effectiveness of Cr immobilization using MALZ composites.
3.4. The impact of adsorption time on Cr immobilization
The impact of adsorption time on the immobilization of e-Cr ions in the CS using thư MALZ was conducted. The experiment involved incubation periods of 15–45 days and the CT (Fig. 5). The experiments utilized artificially Cr-contaminated soils with a 3 % (w/w) mixture weight ratio of MALZ to the CS. The results indicated a consistent decrease in the e-Cr content in the treated soils compared to the CT. Specifically, the percentage of e-Cr forms in the soil decreased from 87.49 % (45.64 mg/kg) in the CT to 40.10 % (20.57 mg/kg) in 15 days, 19.12 % (11.27 mg/kg) in 30 days, and 19.49 % (10.07 mg/kg) in 45 days in the soils treated with MALZ. Furthermore, the majority of the e-Cr form (F1) in the treated soils was transformed into more stable forms, primarily carbonate-bound (F3) and organically complexed (F4), as a result of treatment with MALZ. A secondary transformation occurred, where the e-Cr (F1) was converted into Fe- and Mn- oxide occluded forms (F2), with the remainder being present in the residual form (F5). The findings also revealed that there was no notable distinction in the e-Cr content between the 30-day and 45-day incubation periods, indicating a plateau in immobilization efficiency. This suggests that extending the incubation time beyond 30 days did not significantly decrease the e-Cr content. The transition from the exchangeable to more immobilized forms, such as carbonate-bound and organically complexed, was most pronounced during the initial 30 days of incubation. This increased efficiency in the early stages of the incubation can be ascribed to the plentifulness of active sites on the surfaces of MALZ throughout the 15- and 30-day treatment periods. Subsequently, these active sites likely reached saturation due to the adsorption of e-Cr, leading to a plateau in immobilization efficiency. This research offers important perspectives on the kinetics of Cr immobilization and the time-dependent efficacy of MALZ, highlighting its potential for environmental remediation of Cr-contaminated soils.
Fig. 5.
Influence of incubation time on the transformation of Chromium forms in the CS: Soil pH of 5.0, the SM of 70 % and an initial total Chromium content of 50 mg/kg, MALZ ratio of 3 %.
The study aligns with previous research on the immobilization of HMs in the CS. The study by Iqbal et al. (2016) [39] discovered a reduction in exchangeable Pb using farm manure over a 28-day incubation period. This finding is consistent with the potential of organic amendments, such as farm manure, to immobilize HMs in soil over time [40]. Moreover, the 30-day incubation period was employed to diminish the exchangeable cadmium content in the CS by utilizing Thiourea-modified biochar [41]. Traina & Laperche, (1999) [42] reported that HMs content such as Fe, Mn, Zn, Cd, Cu, and Pb remained almost unchanged using different biochar concentrations at 1.0, 3.0, and 5.0 % in a 30-day incubation time, indicating the need for further investigation into the effectiveness of different biochar application rates in immobilizing HMs in the soil.
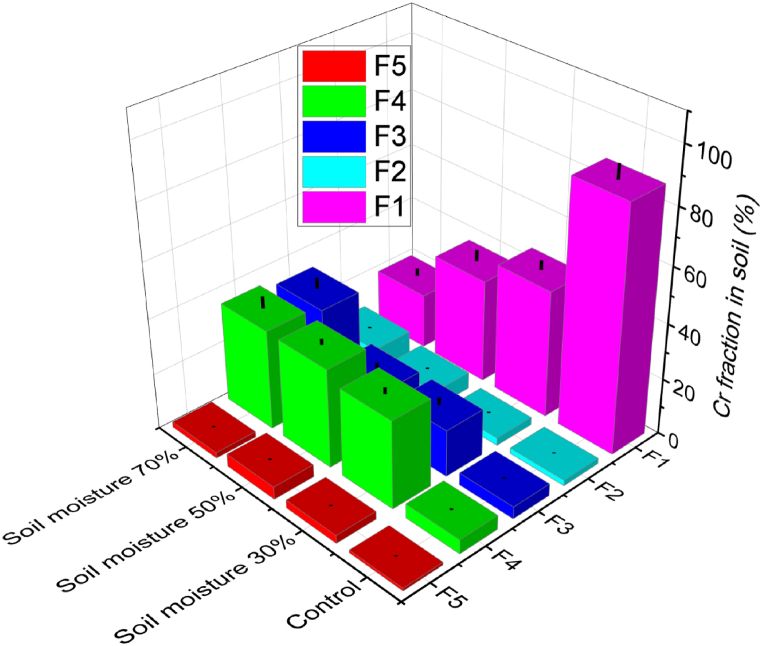
3.5. The impact of SM on Cr immobilization
The impact of varying SM levels on chromium immobilization in the CT was investigated. The experiment involved levels of 30–70 % over a 30-day adsorption time using MALZ, with a constant soil pH of 5.0 (Fig. 6). The CT was also implemented separately to benchmark the efficiency of Cr immobilization under these conditions. The experimental outcomes revealed a clear trend: as the SM increased from 30 % to 70 %, there was a corresponding decrease in the proportion of e-Cr in the soils treated with MALZ. Specifically, the percentage of e-Cr diminished to 45.81 % (23.07 mg/kg), 37.24 % (19.24 mg/kg) and 19.82 % (10.08 mg/kg) for SM content ranging from 30 % to 50 % and 70 %, respectively, after the 30-day incubation. Furthermore, the study observed that the proportion of Cr in various forms – namely, Fe–Mn oxide occluded (F2), carbonate bound (F3), organically complexed (F4), and residual (F5) – elevated by the rising SM levels after the 30-day incubation. These transformed proportions were more pronounced in the soils treated with MALZ than in the CT. Among these forms, the majority of e-Cr was converted into the organically complexed (F4) form, followed by the carbonate bound (F3) form, across all experimental SM conditions (30 %–70 %). The most significant reduction in the e-Cr content was noticed at the SM level of 70 %, which decreased to 19.82 % upon treatment with MALZ. This finding underscores the influential role of SM in facilitating the immobilization of HMs. It highlights the effectiveness of MALZ as an adsorbent under varying moisture conditions.
Fig. 6.
Impact of SM on the transformation of chromium forms in the CS: Soil pH of 5.0, an initial total chromium content of 50 mg/kg, MALZ ratio of 3 %, 30-day incubation.
The significant reduction in the e-Cr content at the SM level of 70 % underscores the influential role of SM in facilitating Cr immobilization. It highlights the effectiveness of MALZ as an adsorbent under varying moisture conditions. This observation aligns with prior research that has emphasized the importance of SM in influencing the efficacy of remediation strategies for soils polluted with HMs [43]. These observations are also in line with prior studies that have shown the impact of SM on the speciation and transformation of HMs in soil, particularly the influence of SM on the formation of different metal forms and complexes ([44].
The observed increase in the immobilization of e-Cr with rising SM can be scientifically rationalized based on several factors affecting the mobility and crystallization of e-Cr ions. One crucial aspect is that elevated SM enhances the interaction between MALZ and e-Cr ions. In this scenario, water serves as a facilitative medium, promoting the free movement of e-Cr ions and enabling more effective contact with MALZ. This heightened interaction between e-Cr ions and the adsorbent leads to improved adsorption and subsequent immobilization of Cr. This phenomenon aligns with earlier studies that have shown the effect of SM on the mobility and speciation of HMs in soil. For instance, reports by Zhang et al. (2018) [22] and Jiang et al. (2013) [45] have highlighted the role of SM in affecting the adsorption and immobilization of HMs in the CS.
Another significant effect of increased moisture is the improvement in the diffusion of Cr ions through the soil matrix. This improved diffusion facilitates a more uniform and effective distribution of Cr ions around adsorbent particles, optimizing adsorption. Consequently, the efficiency of Cr immobilization is observed to rise as SM increases between 30 % and 70 %. Higher SM might induce more negative charges on the surfaces of the adsorbent. This increase in negative charge could reduce exchangeable HMs due to enhanced attraction and binding of positively charged metal ions. In conditions of high SM, there is also a tendency to form organically complexed and carbonate-bound forms of Cr, which further transition into crystalline structures. This transformation indicates the effective immobilization and stabilization of Cr in the soil, reducing its bioavailability and environmental impact. In summary, the study's findings underscore the significant role of SM in influencing the interaction dynamics between Cr ions and adsorbents and the subsequent immobilization and transformation of Cr forms in the soil.
The investigation involved the quantification of Cr(III) and Cr(VI) concentrations in soil samples extracted from the exchangeable solution (F1) both before and after a designated incubation period in Experiment 3 conducted under 70 % SM conditions. The research focused on applying MALZ materials to immobilize chromium in soil, leading to notable observations regarding the transformation of Cr(VI) into Cr(III) across different SM levels. Initially, the soil exhibited a Cr(VI) content of 45.61 mg/kg, representing 99.12 % of the total chromium content in the soil matrix. After a 30-day incubation period with MALZ, significant conversion of Cr(VI) to Cr(III) was evident, as depicted in Table 1. The concentrations of Cr(III) escalated to 28.31 mg/kg, 21.48 mg/kg, and 14.11 mg/kg, corresponding to 86.25 %, 87.19 %, and 89.62 % of the overall chromium content following incubation at SM levels of 30 %, 50 %, and 70 %, respectively. Concurrently, the residual Cr(VI) levels decreased to 4.51 mg/kg, 3.15 mg/kg, and 1.63 mg/kg after the incubation period at SMs of 30 %, 50 %, and 70 %.
Table 1.
The content of Cr(VI) and Cr(II) forms before and in the soil before and after immobilization.
| Total e-Cr (mg/kg) | Cr(VI) (mg/Kg) | Cr(III) (mg/Kg) | |
|---|---|---|---|
| Soil before | 45.64 ± 2.13 | 45.24 ± 2.87 | 0.40 ± 0.03 |
| Soil after incubation at 30 % moisture | 32.82 ± 1.82 | 4.51 ± 0.38 | 28.31 ± 1.48 |
| Soil after incubation at 50 % moisture | 24.60 ± 2.06 | 3.15 ± 0.25 | 21.45 ± 1.83 |
| Soil after incubation at 70 % moisture | 15.74 ± 0.99 | 1.63 ± 0.82 | 14.11 ± 1.06 |
The outcomes of this study signify a successful reduction of Cr(VI) to Cr(III) in the soil samples treated with MALZ. The substantial proportions of Cr(III) detected post-incubation indicate an effective sequestration of chromium within the soil matrix. The conversion of Cr(VI) to Cr(III) holds significance due to the lower toxicity and enhanced immobility of Cr(III) compared to Cr(VI), thereby mitigating the environmental hazards associated with chromium contamination. These findings underscore the efficacy of MALZ materials as promising agents for chromium immobilization in soil, particularly under varying SM conditions.
3.6. Proposed chromium adsorption mechanism
The introduction of MALZ has been found to significantly alter the speciation of chromium in the CS, leading to the transformation of exchangeable forms of Cr into Fe–Mn oxide occluded (F2), carbonate bound (F3), and organically complexed (F4) categories. This alteration underscores the role of MALZ in modifying Cr speciation within a specific time frame. This is supported by research showing that NaY zeolite can effectively extract chromium from diluted solutions, with the structural integrity of the zeolite framework remaining intact post-chromium biosorption [46]. Besides, biogenic manganese (IV) oxides serve as the principal oxidants in these environments, and the reversible nature of chromate reduction in soils can be contingent upon the manganese levels, the redox state of the soils, and the solubility of the chromium (III)-containing phase [47].
The immobilization of HMs in the soil also involves several fundamental processes, including the co-precipitation of chromium (Cr) ions facilitated by the occurrence of iron and aluminum oxyhydroxides in the adsorptive materials (Fig. 7). This mechanism has been supported by evidence from EDS analysis (Fig. 1b1). The observation aligns with previous research that has established the concurrent precipitation of metals alongside iron and manganese oxides in acidic soils. This process is associated with the mobility of these elements. Throughout the adsorption phase, a portion of the Cr(VI) underwent reduction to Cr(III) and subsequently adsorbed onto MALZ. This phenomenon highlights the combined contribution of reduction, ion exchange and adsorption to removing Cr(VI).
Fig. 7.
Schematic of a possible mechanism for the e-Cr reduction utilizing MALZ.
The fixation of e-Cr onto MALZ is significantly influenced by carbonate precipitation within the interlayer regions of the LDH. This process is supported by the work of [48], which demonstrated the leaching of Mg2+ from hydrotalcite and the ready interchange of interlayer carbonate with the adjacent solution, resulting in the generation of uranyl carbonate complexes. Besides, Misol et al. (2022) [49] highlighted the difficulty in preventing the integration of carbonate anions into the interlayer space when using the coprecipitation method, underscoring the strong tendency of carbonate to associate with LDH layers. Moreover, the substantial BET surface area of MALZ (252.66 m2/g) augments its capacity for immobilization through a pore-filling mechanism. Alotaibi and Ismail (2022) [50] emphasized the importance of the particular surface area of natural zeolite in the effective adsorption of thorium (IV), reinforcing the significance of surface area in adsorption processes.
Interlayer ion exchange is a fundamental chemical process that involves the substitution of interlayer anions, such as carbonate ions, with alternative anions, like chromate ions derived from chromium-contaminated water or soil, within the LDH framework. This exchange process is mainly controlled by the electrostatic interaction between the positively charged strata of the LDH and the negatively charged chromate ions. In soil remediation, incorporating Mg/Al LDH into chromium-polluted soil facilitates the displacement of chromate ions by the original carbonate ions, resulting in their presence in the interlayer spaces of LDH. Consequently, this interlayer ion exchange process effectively immobilizes chromium by confining it within the LDH structure. The process of interlayer ion exchange is recognized as a significant pathway for Cr(VI) immobilization. Within the LDH structure, the interlayer anions (e.g., NO₃⁻) undergo exchange with Cr(VI) oxyanions (such as CrO₄2⁻ and Cr₂O₇2⁻) that are prevalent in the soil, leading to the effective immobilization of chromium within the interlayer spaces of LDH. This observation aligns with the findings of Li et al. (2023) [51], who also highlighted that this mechanism serves as a key pathway for the immobilization of Cr(VI), specifically within the LDH structure. The exchange of interlayer anions with Cr(VI) oxyanions plays a crucial role in sequestering chromium and preventing its mobility in the environment. Moreover, the interaction of organic compounds with functional groups such as –C O on the surfaces of MALZ (as shown in Fig. 2b) fosters the formation of carbonate-bound chromium (Cr), enhancing the immobilization capability through electrostatic interaction between carboxylate groups (-C O) and the e-Cr. This interaction results in Mg–Al–CO3–Cr-LDH-zeolite complexes, significantly increasing Cr immobilization and the soil's prevalence of Fe–Mn oxide-bound forms. This finding aligns with prior studies conducted by Li et al. (2023) [52], which demonstrated that the primary mechanisms involved in the immobilization of soil Cr(VI) through the use of LDHs-M are interlayer anion exchange and electrostatic adsorption. The study by Zou et al. (2016) [53] demonstrated the improved coagulation of graphene oxide in solution due to the surface oxygen-bearing groups of LDH-Cl and LDH-CO3 occupying the attachment points of graphene oxide, indicating the significant role of functional groups in interactions with contaminants. The work by Jaloud et al. (2013) [54] highlighted the organic bound fractions of trace elements, with Fe being the dominant fraction, supporting the relevance of organic interactions in trace element immobilization. Furthermore, the study by Liu et al. (2021) [55] revealed the transformation of readily accessible Cr(VI) and total Cr species into less accessible forms of Cr(III) and total Cr, such as species bound to Fe–Mn oxides and organic matter, owing to the formation of Cr–Fe hydroxides and oxides on the FeO surface, emphasizing the role of organic interactions in chromium speciation and immobilization. Another critical mechanism is the reaction between metal ions and the SiO2 component of the adsorbents, leading to the creation of metal silicides and oxides. The research by Ding et al. (2013) [56] showed that exogenous silicon prompted alkalinization in the rhizosphere, facilitating the formation of precipitation-associated and organic matter-associated chromium in chromium-contaminated soil, consequently reducing chromium uptake. Besides, the reactivity of MgO, particularly its rapid dissolution and hydration properties, is advantageous for short-term chromium (Cr) immobilization. The occurrence of magnesium and oxygen elements in MALZ, as depicted in Fig. 1b1, is also pivotal in the immobilization of Cr in the CS.
In conclusion, this comprehensive analysis supports existing literature and deepens our understanding of the intricate mechanisms in soil remediation using MALZ. These insights are vital for devising effective strategies to combat heavy metal pollution in soils, specifically addressing Cr contamination challenges.
In this research, MALZ was utilized for the immobilization of Cr(VI) in soil, achieving an immobilization efficiency of 77.35 %. This efficiency was attained under specific conditions, including a 3 % absorbent-soil ratio (w/w), a Cr concentration of 50 mg/kg, an MS of 70 %, and an incubation time of 30 days (Table 2). The findings of this study offer a novel perspective on the possible use of MALZ for Cr(VI) immobilization in soil environments. Comparative analysis with prior studies reveals that MALZ exhibits superior immobilization efficiency compared to nanoscale Fe/Al-LDH and Fe/Al-LDHs (Table 2), which reported efficiencies of 70 % and 72.45 % respectively, albeit under different experimental settings [57,58]. Notably, the performance of MALZ is particularly remarkable given the relatively low Cr concentration and shorter reaction time in certain instances when contrasted with these previous studies. For example, Nanoscale Fe/Al-LDH was tested under specific mole ratios with the e-Cr content of 38.61 mg/kg. In comparison, Fe/Al-LDHs were applied to soil with a significantly higher Cr(VI) content of 2079.84 mg/kg, albeit with a shorter reaction time of 90 min. In comparison to Mg/Al-LDHs from another study, which exhibited an immobilization efficiency of 62.27 % at a Cr(VI) concentration of 1000 mg/kg in soils, the LDH-zeolite blend in this study demonstrates superior effectiveness under less extreme conditions [59]. However, it is important to note that Fe/Al-LDH-activated carbon and Ca/Al-LDH from other investigations displayed higher efficiencies under their specific conditions, with Fe/Al-LDH-activated carbon achieving an impressive 99.50 % efficiency at a much higher initial Cr(VI) concentration, and Ca/Al-LDH exhibiting efficiencies ranging from 85.50 % to 96.49 %, albeit under longer incubation times and varying Cr concentrations [60,61]. The scientific significance of this study lies in the successful utilization of MALZ as a promising alternative for Cr(VI) immobilization in soils, offering a favorable balance between efficiency and practicality, especially for soils with lower contamination levels. This contribution expands the repertoire of tools available for environmental remediation, particularly in scenarios where other materials may not be as effective or feasible. It underscores the importance of continued exploration of diverse material combinations and conditions to address the multifaceted challenges of the CS.
Table 2.
Compare the Cr immobilization in soil utilizing MALZ and other materials.
| Adsorbents | Experimental conditions | Cr(VI) immobilization efficiency | References |
|---|---|---|---|
| MALZ | Absorbent and soil ratio (w/w): 3 %, Cr concentraton: 50 mg/kg, SM: 70 %, incubation time: 30 days | 77.35 % | This study |
| Nanoscale Fe/Al-LDHs | The mole ratio of Cr(VI) and Fe/Al LDH: 0.2, e-Cr: 38.61 mg/kg | 70 % | [57] |
| Fe/Al-LDHs | Cr(VI) in the soil (2079.84 mg/kg), Fe/Al-LDH and Cr(VI)-contaminated soil ratio of 1: 5, reaction time: 90 min. | 72.45 % | [58] |
| Mg/Al-LDHs | Cr(VI) in soils:1000 mg/kg; Quantity of LDHs: 10 g/L | 62.27 % | [59] |
| Fe/Al-LDH- activated carbon | Quantity of LDHs: 0.05 g; Cr(Ⅵ) content:1000 mg/kg; incubation time: 60 min, pH 4 | 99.50 % | [60] |
| Ca/Al-LDHs | Cr: 200–424 mg/kg, incubation time: 120 h | 85.50 %–96.49 % | [61] |
4. Conclusion
The current study effectively conducted a sequence of tests to explore the influence of multiple variables on the immobilization of e-Cr by incorporating MALZ into the CS. The findings revealed that the optimal conditions for immobilizing the e-Cr using MALZ included a soil pH of 5.0, a MALZ weight ratio of 3 %, the incubation period of 30 days, and an SM level of 70 %. Under these conditions, the most significant reduction in e-Cr content reached 19.82 % (equivalent to 10.08 mg/kg) when employing MALZ. Nearly all of the e-Cr was transformed into various forms, including carbonate-bound (F3), organically complexed (F4), and Fe–Mn oxide occluded (F2), owing to a combination of pore filling, reduction processes, co-precipitation, organic interactions, and electrostatic attraction mechanisms. Therefore, MALZ has demonstrated high efficiency as an environmentally friendly adsorbent with the potential to immobilize the e-Cr in the CS, particularly in acidic soil conditions. However, further research is warranted to address the limitations and fully assess this remediation approach's long-term effectiveness and environmental implications.
Data availability statement
Data associated with this study has not been deposited into a publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Thị Bich Hanh Nguyen: Writing – original draft, Methodology, Investigation, Formal analysis. Huu-Tap Van: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Van Minh Dang: Writing – review & editing, Supervision. Thi Ngoc Ha Tran: Software, Methodology, Conceptualization. Thi Tuyet Nguyen: Resources, Investigation, Data curation. Trung Kien Hoang: Writing – original draft, Software, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was financially supported by the Vietnam Ministry of Education and Training under project number: B2023-TNA-32.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31084.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Nagajyoti P.C., Lee K.D., Sreekanth T.V.M. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010;8:199–216. doi: 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- 2.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S., Cao Q., Zheng Y., Huang Y., Zhu Y. Health risks of heavy metals in contaminated soils and Food Crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008;152:686–692. doi: 10.1016/j.envpol.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan D., Sukla L.B., Sawyer M., Rahman P. Recent Bioreduction of hexavalent chromium in wastewater treatment: a review. J. Ind. Eng. Chem. 2017;55:1–20. doi: 10.1016/j.jiec.2017.06.040. [DOI] [Google Scholar]
- 5.Li H., Yang Y., Zheng W., Chen L., Bai Y. Immobilization of high concentration hexavalent chromium via core-shell structured Lightweight aggregate: a promising soil remediation Strategy. Chem. Eng. J. 2020;401 doi: 10.1016/j.cej.2020.126044. [DOI] [Google Scholar]
- 6.Zhu Y., Song K., Cheng G., Xu H., Wang X., Qi C., Zhang P., Liu Y., Liu J. Changes in the bacterial communities in chromium-contaminated soils. Front. Vet. Sci. 2023;9 doi: 10.3389/fvets.2022.1066048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geelhoed J.S., Meeussen J.C.L., Roe M., Hillier S.J., Thomas R.P., Farmer J.G., Paterson E. Chromium remediation or Release? Effect of iron(II) sulfate addition on chromium(VI) leaching from columns of chromite Ore processing Residue. Environ. Sci. Technol. 2003;37:3206–3213. doi: 10.1021/es0264798. [DOI] [PubMed] [Google Scholar]
- 8.Hu G., He Y., Zhu K., Zhang Z., Lou W., Zhang K., Chen Y.-G., Wang Q. Experimental study on injection of ferrous Sulphate for remediation of a clayey soil contaminated with hexavalent chromium. Environ. Earth Sci. 2023;82:185. doi: 10.1007/s12665-023-10853-y. [DOI] [Google Scholar]
- 9.Sun Y., Guan F., Yang W., Wang F. Removal of chromium from a contaminated soil using oxalic acid, citric acid, and hydrochloric acid: dynamics, mechanisms, and Concomitant removal of Non-targeted metals. Int. J. Environ. Res. Public Health. 2019;16:2771. doi: 10.3390/ijerph16152771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y., Xue F., Muhammad F., Yu L., Xu F., Jiao B., Shiau Y.-C., Li D. Application of iron-loaded activated carbon electrodes for electrokinetic remediation of chromium-contaminated soil in a three-dimensional electrode system. Sci. Rep. 2018;8:5753. doi: 10.1038/s41598-018-24138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M., Gao X., Li C., Yang C., Fu C.-A., Liu J., Wang R., Chen L.-X., Lin J., Liu X., Pang X. Isolation and Identification of chromium reducing Bacillus cereus species from chromium-contaminated soil for the Biological Detoxification of chromium. Int. J. Environ. Res. Public Health. 2020;17:2118. doi: 10.3390/ijerph17062118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Y., Han F.X., Sridhar B.B.M., Monts D.L. Phytotoxicity and Phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ. Toxicol. Chem. 2005;24:2019–2026. doi: 10.1897/04-329r.1. [DOI] [PubMed] [Google Scholar]
- 13.Yang G., Wu H., Ma X., Tang Y., Li J., Chai Y., Wang C., Yang H. Review on the effects of combined pollution of lead and chromium on soil microorganisms and treatment methods. J. Geosci. Environ. Prot. 2020;8:140–150. doi: 10.4236/gep.2020.89009. [DOI] [Google Scholar]
- 14.Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Bagherzadeh M., Aslibeiki B., Arsalani N. Preparation of Fe3O4/Vine Shoots derived activated carbon nanocomposite for improved removal of Cr(VI) from Aqueous solutions. Sci. Rep. 2023;13:3960. doi: 10.1038/s41598-023-31015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Ke X., Wu X., Ke C., Chen R., Chen X., Zheng X., Jin Y., Van der Bruggen B. Simultaneous removal of trivalent chromium and hexavalent chromium from soil using a modified bipolar membrane electrodialysis system. Environ. Sci. Technol. 2020;54:13304–13313. doi: 10.1021/acs.est.0c04105. [DOI] [PubMed] [Google Scholar]
- 17.Mulyanto D., Munawar A., Widodo R.A. Terra Rossa soil as an adsorbent to remove hexavalent chromium in Aqueous solutions. IOP Conf. Ser. Earth Environ. Sci. 2023;1242 doi: 10.1088/1755-1315/1242/1/012018. [DOI] [Google Scholar]
- 18.Ma L., Islam S.M., Liu H., Zhao J., Sun G., Li H., Ma S., Kanatzidis M.G. Selective and efficient removal of toxic oxoanions of as(III), as(V), and Cr(VI) by layered double hydroxide intercalated with MoS42. Chem. Mater. 2017;29:3274–3284. doi: 10.1021/acs.chemmater.7b00618. [DOI] [Google Scholar]
- 19.Zhang R., Zhang N., Fang Z. In situ remediation of hexavalent chromium contaminated soil by CMC-stabilized nanoscale zero-valent iron composited with biochar. Water Sci. Technol. 2018;77:1622–1631. doi: 10.2166/wst.2018.039. [DOI] [PubMed] [Google Scholar]
- 20.Ma L., Wang Q., Islam S.M., Liu Y., Ma S., Kanatzidis M.G. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42– ion. J. Am. Chem. Soc. 2016;1388:2858–2866. doi: 10.1021/jacs.6b00110. [DOI] [PubMed] [Google Scholar]
- 21.Guaya D., Cobos H., Valderrama C., Cortina J.L. Effect of Mn2+/Zn2+/Fe3+ oxy(hydroxide) Nanoparticles doping onto Mg-Al-LDH on the phosphate removal capacity from Simulated wastewater. Nanomaterials. 2022;12:3680. doi: 10.3390/nano12203680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Lei Y., Yuan Y., Gao J., Jiang Y., Xu Z., Zhao S. Enhanced removal performance of Cr(VI) by the core-shell zeolites/layered double hydroxides (LDHs) synthesized from different metal compounds in constructed rapid infiltration systems. Environ. Sci. Pollut. Res. 2018;25:9759–9770. doi: 10.1007/s11356-018-1303-0. [DOI] [PubMed] [Google Scholar]
- 23.yu Shi W., bo Shao H., Li H., an Shao M., Du S. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J. Hazard Mater. 2009;170:1–6. doi: 10.1016/j.jhazmat.2009.04.097. [DOI] [PubMed] [Google Scholar]
- 24.Dang V.M., Van H.T., Vinh N.D., Hoa Duong T.M., Hanh Nguyen T.B., Nguyen T.T., Ha Tran T.N., Hoang T.K., Tran T.P., Nguyen L.H., Chu M.N. Enhancement of exchangeable Cd and Pb immobilization in contaminated soil using Mg/Al LDH-zeolite as an effective adsorbent. RSC Adv. 2021;11:17007–17019. doi: 10.1039/d0ra10530a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian R., Chen D., Liu X., Cui L., Li L., Pan G., Xie D., Zheng J., Zhang X., Zheng J., Chang A. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: results from a cross-site field experiment. Ecol. Eng. 2013;58:378–383. doi: 10.1016/j.ecoleng.2013.07.031. [DOI] [Google Scholar]
- 26.Lian M., Ma Y., Li J., Sun J., Zeng X. Influence of pH on the Particulate-bound Cd speciation and uptake by plants. Polish J. Environ. Stud. 2022;31:5511–5517. doi: 10.15244/pjoes/152224. [DOI] [Google Scholar]
- 27.Wen B., Hu X., Liu Y., Wang W., Feng M., Shan X. The role of earthworms (Eisenia fetida) in influencing bioavailability of heavy metals in soils. Biol. Fertil. Soils. 2004;40:181–187. doi: 10.1007/s00374-004-0761-3. [DOI] [Google Scholar]
- 28.Wang C., Li W., Yang Z., Chen Y., Shao W., Ji J. An invisible soil acidification: critical role of soil carbonate and its impact on heavy metal bioavailability. Sci. Rep. 2015;5:1–9. doi: 10.1038/srep12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Cao J., Zhang W. Stoichiometry of Cr(VI) immobilization using nanoscale zerovalent iron (nZVI): a study with high-Resolution X-ray Photoelectron spectroscopy (HR-XPS) Ind. Eng. Chem. Res. 2008;47:2131–2139. doi: 10.1021/ie061655x. [DOI] [Google Scholar]
- 30.Qin J., Zhao H., Dai M., Zhao P., Chen X., Liu H., Lu B. Speciation distribution and influencing factors of heavy metals in rhizosphere soil of Miscanthus Floridulus in the Tailing Reservoir area of Dabaoshan iron Polymetallic mine in Northern Guangdong. Processes. 2022;10:1217. doi: 10.3390/pr10061217. [DOI] [Google Scholar]
- 31.Naveenkumar T., Backiyavathy M.R., Chitdeshwari T., Maheshwari M., Saraswathi T., Lakshmanan A. Influence of zeolite on heavy metal immobilization in municipal solid waste compost contaminated soil. J. Appl. Nat. Sci. 2022;14:971–977. doi: 10.31018/jans.v14i3.3741. [DOI] [Google Scholar]
- 32.Rossi G., Beni C. Effects of medium-term amendment with diversely processed sewage sludge on soil humification—mineralization processes and on Cu, Pb, Ni, and Zn bioavailability. Plants. 2018;7:16. doi: 10.3390/plants7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budianta W. ICST 2020. 2020. Laboratory study on the use of natural zeolite from Gunungkidul, Indonesia for Cu, Pb, Zn and Cd immobilization in soil; pp. 1–4. [Google Scholar]
- 34.Zanin E., Scapinello J., de Oliveira M., Rambo C.L., Franscescon F., Freitas L., de Mello J.M.M., Fiori M.A., Oliveira J.V., Dal Magro J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017;105:194–200. doi: 10.1016/j.psep.2016.11.008. [DOI] [Google Scholar]
- 35.Yang C.-P., Tseng Y.-L., Chen H.-Y. Kinetics of chromium reduction associated with varying characteristics of Agricultural soils. Water. 2022;14:70. doi: 10.3390/w14040570. [DOI] [Google Scholar]
- 36.Wittbrodt P.R., Palmer C.D. Effect of temperature, ionic Strength, background Electrolytes, and Fe(III) on the reduction of hexavalent chromium by soil humic Substances. Environ. Sci. Technol. 1996;30:2470–2477. doi: 10.1021/es950731c. [DOI] [Google Scholar]
- 37.Zuo X.J., Liu Z., Chen M.D. Effect of H2O2 concentrations on copper removal using the modified hydrothermal biochar. Bioresour. Technol. 2016;207:262–267. doi: 10.1016/j.biortech.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Igalavithana A.D., Kwon E.E., Vithanage M., Rinklebe J., Moon D.H., Meers E., Tsang D.C.W., Ok Y.S. Soil lead immobilization by biochars in short-term laboratory incubation studies. Environ. Int. 2019;127:190–198. doi: 10.1016/j.envint.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal M.M., Hafeez-ur-Rehman, Murtaza G., Naz T., Javed W., Mehdi S.M., Javed S., Sheikh A.A. Transformation and adsorption of lead as affected by organic matter and incubation time in different textured salt-affected soils. J. Agric. Environ. Sci. 2016;1:140–145. [Google Scholar]
- 40.Keesstra S., Bouma J., Wallinga J., Tittonell P., Smith P., Cerdà A., Montanarella L., Quinton J., Pachepsky Y., van der Putten W.H., Bardgett R.D., Moolenaar S.W., Mol G., Jansen B., Fresco L.O. The significance of soils and soil science towards Realization of the United Nations sustainable development Goals. Soil. 2016;2:111–128. doi: 10.5194/soil-2-111-2016. [DOI] [Google Scholar]
- 41.Zhu Y., Ma J., Chen F., Yu R., Hu G., Zhang S. Remediation of soil polluted with cd in a postmining area using thiourea-modified biochar. Int. J. Environ. Res. Public Health. 2020;17:1–14. doi: 10.3390/ijerph17207654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traina S.J., Laperche V. Contaminant bioavailability in soils, Sediments, and Aquatic environments. Proc. Natl. Acad. Sci. 1999;96:3365–3371. doi: 10.1073/pnas.96.7.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Wabel M.I., Usman A.R.A., El-Naggar A.H., Aly A.A., Ibrahim H.M., Elmaghraby S., Al-Omran A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015;22:503–511. doi: 10.1016/j.sjbs.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paz-Ferreiro J., Lu H., Fu S., Méndez A., Gascó G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth. 2014;5:65–75. doi: 10.5194/se-5-65-2014. [DOI] [Google Scholar]
- 45.Jiang H., Deng Q., Zhou G., Hui D., Zhang D., Liu S., Chu G., Li J. Responses of soil Respiration and its temperature/moisture Sensitivity to precipitation in three Subtropical Forests in Southern China. Biogeosciences. 2013;10:3963–3982. doi: 10.5194/bg-10-3963-2013. [DOI] [Google Scholar]
- 46.Silva B., Figueiredo H., Quintelas C., Neves I.C., Tavares T. Zeolites as supports for the Biorecovery of hexavalent and trivalent chromium. Microporous Mesoporous Mater. 2008;116:555–560. doi: 10.1016/j.micromeso.2008.05.015. [DOI] [Google Scholar]
- 47.Thomas A.N., Eiche E., Göttlicher J., Steininger R., Benning L.G., Freeman H.M., Dideriksen K., Neumann T. Products of hexavalent chromium reduction by Green Rust sodium sulfate and associated reaction mechanisms. Soil Syst. 2018;2:58. doi: 10.3390/soilsystems2040058. [DOI] [Google Scholar]
- 48.Foster C., Shaw S., Neill T.S., Bryan N., Sherriff N., Natrajan L.S., Wilson H., Lopez-Odriozola L., Rigby B., Haigh S.J., Zou Y., Harrison R.W., Morris K. Hydrotalcite Colloidal stability and interactions with Uranium(VI) at neutral to alkaline pH. Langmuir. 2022;38:2576–2589. doi: 10.1021/acs.langmuir.1c03179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misol A., Jiménez A., Labajos F.M. Use of Ethylamine, Diethylamine and Triethylamine in the synthesis of Zn,Al layered double hydroxides. Chemengineering. 2022;6:53. doi: 10.3390/chemengineering6040053. [DOI] [Google Scholar]
- 50.Alotaibi A.M., Ismail A.F. Modification of clinoptilolite as a Robust adsorbent for Highly-efficient removal of thorium (IV) from Aqueous solutions. Int. J. Environ. Res. Public Health. 2022;19 doi: 10.3390/ijerph192113774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Fang X., Yuan W., Zhang X., Yu J., Chen J., Qiu X. Preparing of layered double hydroxide- alginate microspheres for Cr(VI)-contaminated soil remediation. Colloids Surfaces A Physicochem. Eng. Asp. 2023;658 doi: 10.1016/j.colsurfa.2022.130655. [DOI] [Google Scholar]
- 52.Li Z., Jing Y., Zhang X., Zhu R., Yu J., Chen J., Qiu X. Recyclable and reusable layered double hydroxide beads for soil remediation: Conveying belt recovery model, mechanism, bioavailability and microbial communities. J. Environ. Chem. Eng. 2023;11 doi: 10.1016/j.jece.2023.110693. [DOI] [Google Scholar]
- 53.Zou Y., Wang X., Ai Y., Liu Y., Li J., Ji Y., Wang X. Coagulation Behavior of graphene oxide on Nanocrystallined Mg/Al layered double hydroxides: Batch experimental and Theoretical Calculation study. Environ. Sci. Technol. 2016;50:3658–3667. doi: 10.1021/acs.est.6b00255. [DOI] [PubMed] [Google Scholar]
- 54.Jaloud A.A.A., Rabhi M., Bashour I. Availability and Fractionation of trace elements in Arid Calcareous soils. Emirates J. Food Agric. 2013;25:702. doi: 10.9755/ejfa.v25i9.14541. [DOI] [Google Scholar]
- 55.Liu L., Zhao J., Yin W., Lv S., Su M., Li P., Zheng X., Chiang P., Wu J. Enhanced immobilization of Cr(VI) by a Fe0–microorganisms composite system: benchmark and Pot experiments. J. Environ. Qual. 2021;50:1123–1134. doi: 10.1002/jeq2.20261. [DOI] [PubMed] [Google Scholar]
- 56.Ding X., Zhang S., Li S., Liao X., Wang G. Silicon Mediated the Detoxification of Cr on Pakchoi (Brassica Chinensis L.) in Cr-contaminated soil. Procedia Environ. Sci. 2013;18:58–67. doi: 10.1016/j.proenv.2013.04.009. [DOI] [Google Scholar]
- 57.Alidokht L., Oustan S., Khataee A., Neyshabouri M.R., Reyhanitabar A. Immobilization of Cr(VI) in soil through injection of nanoscale FeII-AlIII LDH suspension into the soil column. Geoderma. 2020;380 doi: 10.1016/j.geoderma.2020.114648. [DOI] [Google Scholar]
- 58.He X., Zhong P., Qiu X. Remediation of hexavalent chromium in contaminated soil by Fe(II)-Al layered double hydroxide. Chemosphere. 2018;210:1157–1166. doi: 10.1016/j.chemosphere.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Zhao J., Zhang S., Yu Q., Cheng J., Qiu X. Ultrasound-assisted synthesis of single layer MgAl hydrotalcite for the removal of Cr(VI) in solution and soil. Appl. Clay Sci. 2021;204 doi: 10.1016/j.clay.2021.106025. [DOI] [Google Scholar]
- 60.Yuan W., Yu Q., Chen J., Qiu X. Immobilization of Cr(Ⅵ) in polluted soil using activated carbon fiber supported FeAl-LDH. Colloids Surfaces A Physicochem. Eng. Asp. 2022;652 doi: 10.1016/j.colsurfa.2022.129884. [DOI] [Google Scholar]
- 61.Xu Y., Xia W., Hou H., Zhang J., Qian G. Remediation of chromium-contaminated soil by electrokinetics and electrokinetics coupled with CaAl-LDH permeable reaction barrier. Environ. Sci. Pollut. Res. 2017;24:20479–20486. doi: 10.1007/s11356-017-9705-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has not been deposited into a publicly available repository. Data will be made available on request.