Abstract
A mutation in the steroidogenic acute regulatory protein (STAR) gene, which encodes a protein that plays a crucial role in steroid hormone synthesis, causes a severe form of congenital adrenal hyperplasia (CAH) known as lipoid CAH (LCAH). LCAH presents with primary adrenal insufficiency (PAI) as well as atypical genitalia. Individuals with LCAH require adrenal steroid hormone supplements for survival. Masculinization in males with STAR deficiency varies from incomplete to normal virilization. Radiological examinations reveal enlarged and lipid-laden adrenals.
A 10-year-old boy born of second-degree consanguinity presented with weight gain and hyperpigmentation for 1 year. He was diagnosed with PAI at age 7 months and treated with hydrocortisone and fludrocortisone. Dynamic adrenal gland testing revealed undetectable hormone reserves. Imaging detected hypoplastic adrenals and a small testis with testicular adrenal rests (TART). Genetic analysis indicated a novel homozygous pathogenic variant of STAR in exon 7, c.814C > G(pArg272Gly) associated with LCAH (OMIM No. 201710). Testing revealed that asymptomatic family members and relatives were heterozygotes for the variant. The patient was diagnosed with nonclassic LCAH with hypoplastic adrenals and TART. Adequate hormone supplementation resulted in TART regression. This genetic variation is reported for the first time.
Keywords: lipoid congenital adrenal hyperplasia, primary adrenal insufficiency, testicular adrenal rests, adrenal hypoplasia, disorders of sexual differentiation, STAR gene
Introduction
Lipoid congenital adrenal hyperplasia (LCAH) is a rare autosomal recessive disorder caused by a steroidogenic acute regulatory protein (STAR) mutation (1). Adrenal steroid hormone production is impaired both in adrenal and gonadal cells with cholesterol ester accumulation in the cell cytosol. The human STAR protein facilitates cholesterol movement from the outer to the inner mitochondrial membrane to be converted to pregnenolone by P450SCC (side chain cleavage, CYP11A1). Affected children exhibit primary adrenal insufficiency (PAI). A majority of 46 XY individuals with classic LCAH (CLCAH) are typically reared as females because of the absence of virilization and undescended testis, which are frequently located in the abdomen. Imaging reveals enlarged and lipid-rich adrenals (2).
LCAH cases are reported mainly in people of Japanese, Korean, and Palestinian descent (3, 4). The STAR-Gln258* mutation with complete loss of function is identified as a founder effect in Korea and other East Asian countries (4).
Case Presentation
A 10-year-old boy presented with excessive weight gain (4 kg in 1 year), extreme fatigue, and skin hyperpigmentation over the extensor aspect of joints on the hands, elbows, and knees. He was the youngest of 3 children and was born at term with second-degree consanguinity (Fig. 1). He cried soon after birth (birth weight of 2 kg). The delivery and postnatal period were uneventful. The small gestational age indicated no identifiable cause. At age 7 months, he was hospitalized for fever and recurrent vomiting and was diagnosed with PAI (Table 1). He was treated with hydrocortisone and fludrocortisone. His records revealed persistently increased adrenocorticotropin (ACTH) and decreased cortisol levels. His eldest sibling died at age 2 years of fever and hypotension. The second pregnancy resulted in an abortion at 3 months. The third sibling is aged 12 years and asymptomatic.
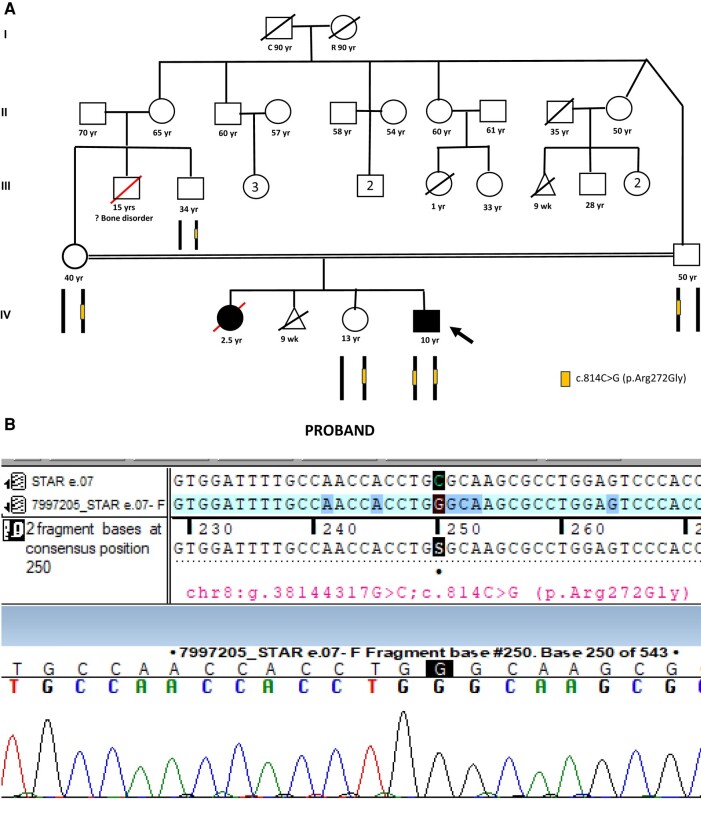
Figure 1.
A, Consanguineous pedigree showing heterozygous unaffected parents and sister. Homozygous affected proband. B, Sanger sequence of a proband with the chromatogram.
Table 1.
Hormonal profile from time of diagnosis (aged 7 months) until presentation to our department February 21, 2023
| Test | Normal range | 27-May 13 | 31-Aug 13 | 30-Nov 13 | 14-Jun 14 | 16-Dec 14 | 19-Nov 16 | 14-Mar 20 | 06-Mar 21 | 10-Mar 22 | 21-Feb-23 | 9-Mar 23 | 13-Sep 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTH 8 Am | 6-46 pg/mL | >1250 pg/mL | 351 pg/mL | 11.3 pg/mL | 591 pg/mL | 19 pg/mL | 623 pg/mL | 1546 pg/mL | 218.8 pg/mL | 570 pg/mL | 752.1 pg/mL | 63.67 pg/mL | 51.05 pg/mL |
| (1.32-10.12 pmol/L) | (>275.2 pmol/L) | (77.29 pmol/L) | (2.49 pmol/L) | (130.14 pmol/L) | (4.18 pmol/L) | (137.19 pmol/L) | (340.43 pmol/L) | (48.18 pmol/L) | (125.52 pmol/L) | (165.612 pmol/L) | (14.02 pmol/L) | (11.24 pmol/L) | |
| Serum cortisol (8 Am) | 6.7-22.6 mcg/dL | 26 mcg/dL | 1.9 mcg/dL | 0.19 mcg/dL | |||||||||
| (184.9-623.8 nmol/L) | (717.6 nmol/L) | (52.44 nmol/L) | (5.244 nmol/L) | ||||||||||
| Serum sodium | 133-143 mmol/L | 138 mmol/L | 135 mmol/L | 131 mmol/L | 137 mmol/L | 140 mmol/L | 136 mmol/L | 136 mmol/L | 141 mmol/L | 140 mmol/L | 137 mmol/L | — | 137.4 mmol/L |
| (133-143 mEq/L) | (138 mEq/L) | (135 mEq/L) | (131 mEq/L) | (137 mEq/L) | (140 mEq/L) | (136 mEq/L) | (136 mEq/L) | (141 mEq/L) | (140 mEq/L) | (137 mEq/L) | — | (137.4 mEq/L) | |
| Serum potassium | 3.5-5.1 mmol/L | 4.9 mmol/L | 4.3 mmol/L | 4.8 mmol/L | 4.5 mmol/L | 4.5 mmol/L | 4.81 mmol/L | 4.1 mmol/L | 4.53 mmol/L | 4.2 mmol/L | 4.6 mmol/L | — | 5 mmol/L |
| (3.5-5.1 mEq/L) | (4.9 mEq/L) | (4.3 mEq/L) | (4.8 mEq/L) | (4.5 mEq/L) | (4.5 mEq/L) | (4.81 mEq/L) | (4.1 mEq/L) | (4.53 mEq/L) | (4.2 mEq/L) | (4.6 mEq/L) | — | (5 mEq/L) |
Abbreviation: ACTH, adrenocorticotropin.
Physical examination revealed a height and weight of 141 cm and 61.5 kg, respectively, with a body mass index of 30.93. His height was in the 50th to 70th percentile and his weight was greater than the 97th percentile (Indian Academy of Paediatrics growth charts). His father's and mother's heights were 168 and 163 cm, respectively. His pulse rate and blood pressure were 89 beats/min and 114/66 mm Hg, respectively. Axillary and pubic hair were absent. The scrotum was well developed with rugosity and mild pigmentation, and testes were descended on both sides, with a volume of 3 mL (Prader orchidometer) and a phallic length of 3 cm (pubertal stage Tanner stage 1). The systemic examination indicated normal findings.
Diagnostic Assessment
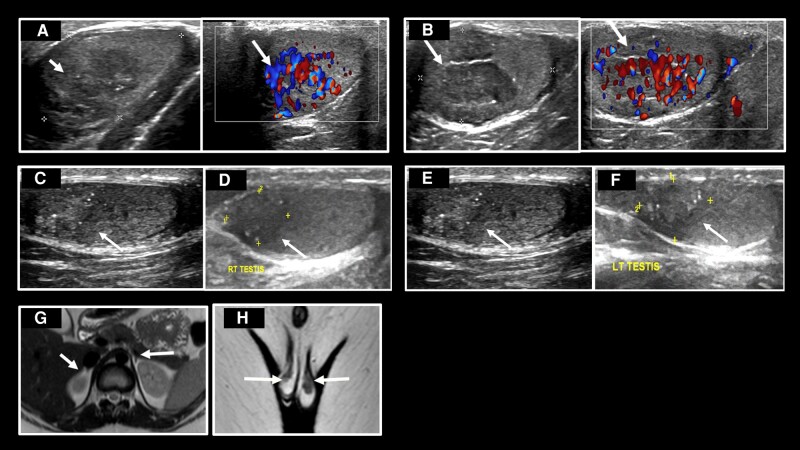
Adrenal reserve testing was not previously recorded. Hydrocortisone was changed over to dexamethasone for adrenal reserve testing. Dynamic testing detected no basal and ACTH-stimulated adrenal glucocorticoid and mineralocorticoid steroid reserves (Table 2). The human chorionic gonadotropin test indicated subnormal testicular function (Table 3). Ultrasound of the scrotum revealed hypoechoic tissue in the parenchyma of the normal testes, demonstrating increased vascularity on Doppler images, indicating testicular adrenal rests (TART) (Fig. 2A and 2B). Magnetic resonance imaging (MRI) axial T2-weighted images revealed hypoplastic adrenals (Fig. 2G). Scrotum MRI revealed T2 hypointense lesions in both testes, indicating TART (Fig. 2H).
Table 2.
Adrenocorticotropin stimulation testing for adrenal reservea
| Tests | Normal range | Basal | |
|---|---|---|---|
| 17 OH progesterone | ng/mL | 0.03-0.90 ng/mL | 0.03 ng/mL |
| (nmol/L) | (0.09-2.72 nmol/L) | (0.09 nmol/L) | |
| 11 Deoxy cortisol | ng/mL | 0.2-1.58 ng/mL | 0.02 ng/mL |
| (nmol/L) | (0.58-4.57 nmol/L) | (0.058 nmol/L) | |
| Cortisol | mcg/dL | 6.7-22.6 mcg/dL | <0.16 mcg/dL |
| (nmol/L) | (184.9-623.8 nmol/L) | (<4.42 nmol/L) | |
| Progesterone | ng/mL | 0.10-0.84 ng/mL | <0.08 ng/mL |
| (nmol/L) | (0.32-2.67 nmol/L) | (<0.25 nmol/L) | |
| 11 Deoxy corticosterone | ng/dL | < 31 ng/dL | <16 ng/dL |
| (nmol/L) | (<0.91 nmol/L) | (<0.48 nmol/L) | |
| Aldosterone | ng/dL | 2.52-39.2 ng/dL | <0.97 ng/dL |
| (nmol/L) | (0.07-1.09 nmol/L) | (<0.027 nmol/L) | |
| Renin | ng/mL/h | 0.5-3.3 ng/mL/h | 2.57 ng/mL/h |
| (mcg/L/h) | (0.5-3.3 mcg/L/h) | (2.57 mcg/L/h) | |
| DHEAS | mcg/dL | 24.40-247.00 mcg/dL | 0.1 mcg/dL |
| μmol/L | (0.66-6.7 μmol/L) | (0.0027μmol/L) | |
| Androstenedione | ng/mL | 0.7-3.6 ng/mL | <0.3 ng/mL |
| (nmol/L) | (2.44-12.57 nmol/L) | (<1.05 nmol/L) | |
| Total testosterone | ng/mL | 0.07-1.3 ng/mL | <0.49 ng/mL |
| (pmol/L) | (242-4420 pmol/L) | (<169.9 pmol/L) |
Abbreviation: DHEAS, dehydroepiandrosterone sulfate.
a Values at 30 and 60 minutes were no different from the basal values.
Table 3.
Human chorionic gonadotropin test to evaluate testicular function on the 5th and 30th day
| Tests | Normal range | Basal | 6th d | 30th d | |
|---|---|---|---|---|---|
| Total testosterone | ng/mL | 0.07-1.3 ng/mL | <0.049 ng/mL | 0.27 ng/mL | 0.35 ng/mL |
| (pmol/L) | (242-4420 pmol/L) | (<166.6 pmol/L) | (918 pmol/L) | (1190 pmol/L) | |
| Free testosterone | pg/mL | 1.3-55.2 pg/mL | 0.69 pg/mL | 0.83 pg/mL | 1.88 pg/mL |
| (pmol/L) | (4.5-191.4 pmol/L) | (2.39 pmol/L) | (2.88 pmol/L) | (6.52 pmol/L) | |
| DHEAS | μg/dL | 24.4-247 μg/dL | <0.1 μg/dL | — | 1.4 μg/dL |
| (μmol/L) | (0.66-6.70 μmol/L) | (<0.003μmol/L) | (0.003 μmol/L) | ||
| Androstenedione | ng/mL | 0.7-3.6 ng/mL | <0.3 ng/mL | — | <0.3 |
| (nmol/L) | (2.44-12.57 nmol/L) | (<1.05 nmol/L) | (<0.3 nmol/L) | ||
| AMH | ng/mL | 8.9-109 ng/mL | >23 ng/mL | — | — |
| (pmol/L) | (63.6-778.6 pmol/L) | (>164 pmol/L) | |||
| Inhibin | pg/mL | 169-216 pg/mL | 112.47 pg/mL | — | — |
| ng/L | (169-216 ng/L) | (112.47 ng/L) | — | — |
Abbreviation: AMH, antimüllerian hormone; DHEAS, dehydroepiandrosterone sulfate.
Figure 2.
A, Ultrasound and Doppler images of right testis showing adrenal rest (white arrows), size 11.2 × 10 mm. B, Ultrasound and Doppler images of left testis showing adrenal rest (white arrows), size 12.5 × 8 mm. C, Ultrasound image of right testis 6 months later, adrenal rest (white arrow) size 10 × 6.5 mm. D, Ultrasound image of right testis 8 months later, adrenal rest (white arrow) size 10 × 6.2 mm. E, Ultrasound image of left testis 6 months later, adrenal rest (white arrow) size 8 × 5.6 mm. F, Ultrasound image of left testis 8 months later, adrenal rest (white arrow) size 6.9 × 4 mm. G, Magnetic resonance image (MRI) T2W axial image of adrenal glands—very small glands—white arrows. H, MRI T2W coronal image of scrotum—T2 hypointense adrenal rests in both testes (white arrows).
Treatment
ACTH levels were suppressed to normal after initiating hydrocortisone of 10 mg/m2/day in the evening and fludrocortisone of 0.1 mg/m2/day.
Outcome and Follow-up
Hyperpigmentation and fatigue were improved. Repeated ultrasound at 6 months (Fig. 2C and 2D) and 8 months (Fig. 2E and 2F) revealed decreased size and increased calcification of TART.
Clinical exome sequencing by next-generation sequencing by an outsourced College of American Pathologists–certified commercial laboratory (MEDGENOME-CLIA–certified laboratory) revealed a homozygous variant in the STAR gene at exon 7, c.814C > G(pArg272Gly)(SCV004042697.1) in the patient (5). STAR gene mutations are associated with LCAH (OMIM No. 201710). The STAR gene at exon 7, c.814C > G(pArg272Gly) homozygous variant has not been reported in the 1000 Genome, Genome Aggregation Database (v3.1), and TOPMed databases and has demonstrated a minor allele frequency of 0.002% in the company's database. The homozygous variant is categorized as a variant of unknown significance as per the American College of Medical Genetics and Genomics–Association for Molecular Pathology criteria. The in silico predictions by PolyPhen-2 (HumDiv), Scale-Invariant Feature Transform, Likelihood Ratio Test, and Mutation Taster 2 of the variant are damaging. The reference codon is conserved across species. The asymptomatic family members demonstrated a heterozygous variant. Fig. 1B shows the chromatogram of the patient.
Discussion
A 10-year-old boy was born at term of second-degree consanguineous marriage and was on treatment for PAI since childhood. He presented with weight gain and hyperpigmentation. Dynamic testing revealed no adrenal reserve. Radiological evaluation indicated hypoplasia of the adrenals and TART. Genetic testing revealed a novel homozygous missense variant of the STAR gene at exon 7, c.814C > G(pArg272Gly-homozygous) (SCV 004042697.1) (5), with his family members carrying the heterozygous form. The homozygous nature of the variant and bioinformatics studies indicate that this could be the causative variant, although there is no functional evidence to confirm this causality.
Human STAR is located on chromosome 8p11.2 and consists of 7 exons that translate into a protein of 285 amino acids (6). The amino acid sequence 67 to 280 of the STAR protein is highly conserved. The mutations may be missense or frameshift mutations. Missenses are the most prevalent, followed by frameshift, splicing, and nonsense mutations (3, 6). Missense mutations occur in the region between 169 and 275 amino acid sequences and are clustered in exons 5 to 7, while frameshift mutations are observed throughout the gene (7). Nonsense or frameshift mutations are determined only in CLCAH (7).
The literature indicates either homozygous or compound heterozygous DNA variants that result in the CLCAH phenotype in the STAR gene at the Gln258 region. So far, the variants described result in the following DNA variants and amino acid changes in various populations globally: STAR gene (a) exon 7, c.898C > T(p.Q258X) in Japan and Korea; (b) exon 5, c.671G > T(A182L) in Palestinian Arabs; (c) exon 5, c.431T > G(p.R182H) in Saudi Arabia; (d) exon 3 c.229C > T(p.Q77X) and exon 7.c. 722C > T(p.Q258X) in China; (e) exon 5, c.444C > A(p.N148 K) and exon 5, c.557C > T(p.R193X) in White individuals (2, 5, 8, 9). NCLCAH demonstrated only a partial loss-of-function mutation.
Here, we report a novel missense variant in STAR gene exon 7, c.814C > G(pArg272Gly) (homozygous) of CLAH (SCV.004042697.1) (5). A missense mutation at the same genomic coordinates as ours with the amino acid cysteine in STAR (−) c.814C > T(p.Arg272Cys) has been reported in Japan (2, 3). All the patients in this group had complete male external genitalia and had pubertal development without androgen supplementation. However, this study mentioned no radiological appearance of the adrenals. Studies in India have revealed 2 novel mutations: premature termination STAR(–) exon 4, c.441G > A (p.W147X), a missense mutation STAR(–) exon 6, c.653C > T (p.A218V), and frameshift deletion c.del 815 G (or p.R272PfsX35). All of these 3 patients had 46 XY karyotypes with female external genitalia (10). Another study from India of patients with PAI and inguinal testis revealed a homozygous mutation STAR(–) exon 4, c.441G > A (p.W147X) (10).
The disrupted gene of LCAH is expressed only in the gonads and adrenals but not in the placenta. The C-terminal region is the biologically important cholesterol-binding site, and the N-terminal region is the mitochondrial target sequence. STAR deletion in the N-terminal region does not cause loss of function, whereas STAR deletion in the C-terminal region (crucial for activity) causes severe functional defects. The 2-hit model explains the pathophysiology; the first hit demonstrated the loss of the STAR-mediated acute steroidogenic response, causing an 80% decrease in STAR-dependent steroidogenesis. Intracellular cholesterol esters and oxidation product accumulation characterize STAR-independent steroidogenesis. In the second hit, the cells engorge with the accumulation of these products, damaging the cytoarchitecture through biochemical and physical displacement (2, 3).
Adrenal and gonadal steroid deficiencies related to lipid accumulation cause problems in patients with LCAH (2, 3). Neonatal salt-wasting, hyperkalemia, hypovolemia, dehydration, acidosis, female external genitalia in 46 XY, and death in infancy are the clinical descriptions of CLCAH (3, 10). Hyperpigmentation due to ACTH hypersecretion is observed in two-thirds of infants. The 46 XY infants have impairments in testosterone synthesis. LCAH treatment typically involves deficient hormone replacement. Adequate supplements of steroids cause hyperpigmentation reduction and TART regression (Fig. 2C-2F).
LCAH is categorized into classic and nonclassic forms (Table 4) (2). The onset of PAI depends on the degree and timing of stress that the patient encounters. CLCAH and NCLCAH demonstrate the absence of adrenal enlargement (2, 11). Adrenal hypoplasia in NCLCAH has been reported in Chinese patients (1, 12).
Table 4.
Lipoid congenital adrenal hyperplasia (2)
| CLCAH | NCLCAH | |
|---|---|---|
| Age of onset of PAI | Neonatal period | Late onset of PAI 1 y or older |
| Skin hyperpigmentation | Present (90%) | Present (100%) |
| External genitalia | Female or minimally masculinized genitalia, irrespective of chromosomal sex | Completely masculinized male external genitalia with karyotype 46XY |
| Plasma ACTH | High | High |
| Serum cortisol | Low | Low |
| Serum Aldosterone | Low | Preserved mineralocorticoid function |
| Plasma renin | Higher | Lower compared to CLCAH |
| Hyponatremia | Present | Present |
| Hypoglycemia | Present | Present |
| Radiology | Lipoid-laden adrenals by CT (68%) | Lipoid-laden adrenals by CT (40%) |
| Pathogenic variant of STAR | Complete loss of function | Partial loss of function |
| Spontaneous puberty | Yes in 90% of XX females | Yes in 100 XY males and XX females |
| Glucocorticoid supplementation | 100% | 100% |
| Mineralocorticoid supplementation | 100% | 64% |
Abbreviations: ACTH, adrenocorticotropin; CLCAH, classic lipoid congenital adrenal hyperplasia; CT, computed tomography; LCAH, lipoid congenital adrenal hyperplasia; NCLCAH, lipoid congenital adrenal hyperplasia; PAI, primary adrenal insufficiency; STAR, steroidogenic acute regulatory protein.
LCAH should be differentiated from other glucocorticoid and mineralocorticoid deficiencies. Enzymatic defects in steroidogenesis can present as PAI in a neonate and the first year of life. Precursor hormones preceding the enzymatic block in CAH are elevated and the adrenals are visualized and may be bulky. Congenital adrenal hypoplasia (X-linked adrenal hypoplasia congenita), where the adrenals are not visualized by imaging methods, is the most difficult to differentiate (3). Cholesterol biochemistry defects, peroxisomal problems, and mitochondrial disorders are associated with skeletal abnormalities and other endocrinopathies (13). Other causes of PAI are IMAGe syndrome with immunodeficiency, where adrenal hypoplasia is present. However, these conditions exhibit hypogonadotropic hypogonadism and skeletal changes (13).
Patients with CAH experience TART, with a prevalence of 40% (14). TART is always bilateral and asymptomatic. TART originates from pluripotent cells of the urogenital ridge, or adrenogonadal primordium. Aberrant adrenal cells end up within the testis or ovaries, which usually regress within the first year of life. ACTH receptors are found in TART (14). Elevated ACTH and increased growth-promoting factors are crucial stimulating factors of growth and hyperplasia of TART. This is supported by the high and low prevalence of TART in severe and nonclassic CAH (moderate ACTH elevation), respectively (14). High ACTH levels in this patient explain the TART.
Moreover, TART exhibits testicular characteristics. The pubertal rise in luteinizing hormone causes TART cell proliferation as luteinizing hormone receptors are also found in TART (15). Bilateral presentation helps to differentiate them from testicular Leydig cell tumors. Intensified glucocorticoid treatment may reduce the TART size (14) as seen in our patient. The presence of TART in NCLCAH remains unreported in the literature.
Therefore, the present STAR gene exon 7, c.814C > G(pArg272Gly) variant may be related to a continuum of the spectrum between NCLCAH and CAH. The novel variant of NCLCAH with hypoplastic adrenals and TART is an unusual finding. The major limitation of this case report was the rarity of the case and the lack of financial wherewithal to complete the functional studies for this variant.
Learning Points
PAI in the neonatal and early-infancy periods should be investigated for enzymatic defects in adrenal steroidogenesis.
Patients with CLCAH and NCLCAH require adrenal steroid hormone replacement therapy for survival.
Adrenals and gonadal imaging is important in patients with LCAH.
Optimal adrenal steroid hormone replacement therapy reverses TART in patients with LCAH.
Monitoring growth and height is important while receiving adrenal steroid replacement in patients with LCAH.
Acknowledgments
The authors express their gratitude to the Sakra administration for all the help in the clinical workup and publication; to Dr Khushal Shah, Department of Laboratory Medicine, Sakra Hospitals, Bangalore, for his coordination of laboratory investigations; Dr G. Kumaramanickavel for his meticulous review and comments on this article; and Dr Brinda Ramanathan for her help in the registration of the novel variant on the National Centre for Biotechnology Information website, both from GenVams Trust, Chennai.
Abbreviations
- ACTH
adrenocorticotropin
- CAH
congenital adrenal hyperplasia
- CLCAH
classic lipoid congenital adrenal hyperplasia
- LCAH
lipoid congenital adrenal hyperplasia
- MRI
magnetic resonance imaging
- NCLCAH
lipoid congenital adrenal hyperplasia
- PAI
primary adrenal insufficiency
- STAR
steroidogenic acute regulatory protein gene
- TART
testicular adrenal rests
Contributor Information
Chittari Venkata Harinarayan, Institute of Endocrinology, Diabetes, Thyroid and Osteoporosis Disorders, Sakra World Hospitals, Bangalore 560103, Karnataka State, India; Department of Medicine & Endocrinology, Saveetha Institute of Medical and Technical Sciences University, Saveetha Medical College, Chennai 600077, India.
Halkurke Shivashankariah Vikram, Department of Internal Medicine, Sakra World Hospitals, Bangalore 560103, Karnataka State, India.
Anisha Sawkar Tandon, Department of Radiology, Sakra World Hospitals, Bangalore 560103, Karnataka State, India.
Marsha Zacharia, Institute of Endocrinology, Diabetes, Thyroid and Osteoporosis Disorders, Sakra World Hospitals, Bangalore 560103, Karnataka State, India.
Shabnam Roohi, Department of Laboratory Medicine, Sakra World Hospitals, Bangalore 560103, Karnataka State, India.
Raghu Janardhan, Department of Internal Medicine, Sakra World Hospitals, Bangalore 560103, Karnataka State, India.
Contributors
C.V.H. designed the clinical evaluation, investigation, genetic studies, literature search, and write-up of the manuscript. H.S.V. and M.Z. helped in adrenal dynamic testing and clinical evaluation. A.S.T. participated in radiological evaluation. S.R. participated in laboratory investigations. R.J. participated in clinical evaluation.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient's relatives or guardians.
Data Availability Statement
Original data generated and analyzed for this case report are included in this published article.
References
- 1. Prader A, Siebenmann RE. Nebennierenisuffizienz bei kongenitaler Lipoid hyperplasie der Nebennieren [Adrenal insufficiency in congenital lipoid hyperplasia of the adrenals]. Helv Paediatr Acta. 1957;12(6):569‐595. [PubMed] [Google Scholar]
- 2. Ishii T, Tajima T, Kashimada K, et al. Clinical features of 57 patients with lipoid congenital adrenal hyperplasia: Criteria for Nonclassic Form Revisited. J Clin Endocrinol Metab. 2020;105(11):dgaa557. [DOI] [PubMed] [Google Scholar]
- 3. Bose HS, Sugawara T, Strauss JF III, Miller WL; International Congenital Lipoid Adrenal Hyperplasia Consortium . The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335(25):1870‐1878. [DOI] [PubMed] [Google Scholar]
- 4. Kang E, Kim YM, Kim GH, Lee BH, Yoo HW, Choi JH. Mutation spectrum of STAR and a founder effect of the p.Q258* in Korean patients with congenital lipoid adrenal hyperplasia. Mol Med. 2017;23(1):149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Center for Biotechnology Information . ClinVar; [VCV002626753.1]. Accessed March 18, 2024. https://www.ncbi.nlm.nih.gov/clinvar/variation/2626753/?utm_source=clinsig_notification&utm_medium=email&utm_campaign=clinsig_updates
- 6. Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85(10):3636‐3639. [DOI] [PubMed] [Google Scholar]
- 7. Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165(Pt A):18‐37. ISSN 0960-0760. [DOI] [PubMed] [Google Scholar]
- 8. Huang Z, Ye J, Han L, et al. Identification of five novel STAR variants in ten Chinese patients with congenital lipoid adrenal hyperplasia. Steroids. 2016;108:85‐91. [DOI] [PubMed] [Google Scholar]
- 9. Khoury K, Barbar E, Ainmelk Y, Ouellet A, Lavigne P, LeHoux JG. Thirty-eight-year follow-up of two sibling lipoid congenital adrenal hyperplasia patients due to homozygous steroidogenic acute regulatory (STARD1) protein mutation. Molecular structure and modeling of the STARD1 L275P mutation. Front Neurosci. 2016;10:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasudevan L, Joshi R, Das DK, et al. Identification of novel mutations in STAR gene in patients with lipoid congenital adrenal hyperplasia: a first report from India. J Clin Res Pediatr Endocrinol. 2013;5(2):121‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joshi R, Das D, Tamhankar P, Shaikh S. Phenotypic variability in congenital lipoid adrenal hyperplasia. Indian Pediatr. 2014;51(5):399‐400. [PubMed] [Google Scholar]
- 12. Zhao X, Su Z, Liu X, et al. Long-term follow-up in a Chinese child with congenital lipoid adrenal hyperplasia due to a STAR gene mutation. BMC Endocr Disord. 2018;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nisticò D, Bossini B, Benvenuto S, Pellegrin MC, Tornese G. Pediatric adrenal insufficiency: challenges and solutions. Ther Clin Risk Manag. 2022;18:47‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engels M, Span PN, van Herwaarden AE, Sweep FCGJ, Stikkelbroeck NMML, Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr Rev. 2019;40(4):973‐987. [DOI] [PubMed] [Google Scholar]
- 15. Claahsen-van der Grinten HL, Hermus AR, Otten BJ. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2009;2009(1):624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed for this case report are included in this published article.