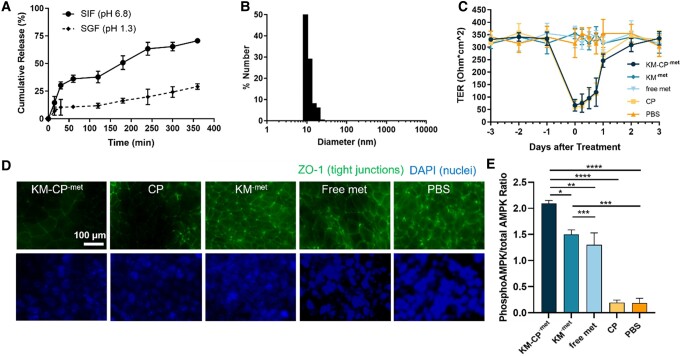
Fig. 3.
In vitro therapeutic efficacy of KM-CP-met. A) In vitro release of KM from KM-CP under pH conditions found in the GI tract (pH = 1.3, SGF; 6.8 SIF, N ≥ 4). B) DLS size measurements of micelles released from SIF at 3 h. C) TER measurements of Caco-2 monolayers incubated with KM-CP-met, KM-met, free metformin, CP, or PBS for up to 6 days. D) Caco-2 monolayers stained with ZO-1 and DAPI at 6 h showed a decrease in ZO-1 signal in treatment groups that contained chitosan. E) Phosphorylated AMPK to total AMPK obtained via ELISA on mpkCCDc14 cells to test the therapeutic efficacy of KM-CP-met (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, N ≥ 3).