Abstract
Background
Deforestation is an important driver of malaria dynamics, with a relevant impact on mosquito ecology, including larval habitat availability, blood-feeding behaviour, and peak biting time. The latter is one of several entomological metrics to evaluate vectorial capacity and effectiveness of disease control. This study aimed to test the effect of forest cover percentage on the peak biting time of Plasmodium-uninfected and infected Nyssorhynchus darlingi females.
Methods
Mosquitoes were captured utilizing human landing catch (HLC) in the peridomestic habitat in field collections carried out in the wet, wet-dry transition, and dry seasons from 2014 to 2017 in areas with active malaria transmission in Amazonian Brazil. The study locations were in rural settlements in areas with the mean annual malaria parasite incidence (Annual Parasite Incidence, API ≥ 30). All Ny. darlingi females were tested for Plasmodium spp. infection using real time PCR technique. Forest cover percentage was calculated for each collection site using QGIS v. 2.8 and was categorized in three distinct deforestation scenarios: (1) degraded, < 30% forest cover, (2) intermediate, 30–70% forest cover, and (3) preserved, > 70% forest cover.
Results
The highest number of uninfected female Ny. darlingi was found in degraded landscape-sites with forest cover < 30% in any peak biting time between 18:00 and 0:00. Partially degraded landscape-sites, with (30–70%) forest cover, showed the highest number of vivax-infected females, with a peak biting time of 21:00–23:00. The number of P. falciparum-infected mosquitoes was highest in preserved sites with > 70% forest cover, a peak biting at 19:00–20:00, and in sites with 30–70% forest cover at 22:00–23:00.
Conclusions
Results of this study show empirically that degraded landscapes favour uninfected Ny. darlingi with a peak biting time at dusk (18:00–19:00), whereas partially degraded landscapes affect the behaviour of Plasmodium-infected Ny. darlingi by shifting its peak biting time towards hours after dark (21:00–23:00). In preserved sites, Plasmodium-infected Ny. darlingi bite around dusk (18:00–19:00) and shortly after (19:00–20:00).
Keywords: Mosquito behavior, Malaria, Entomological surveillance, Deforestation, Land use change
Background
The Amazon River basin is the location of the largest tropical rainforest in the world, with the highest biodiversity and renewable water resources [1]. However, the woodland has been losing its cover to logging, extensive agribusiness, legal and illegal mining, urbanization, and construction of infrastructure [2, 3]. With continuous severe land-use change, inhabitants of these areas are constantly at risk of acquiring malaria [4]. The occurrence of malaria shows a spatiotemporal heterogeneity associated with several components that include those of the mosquito vectors, Plasmodium parasite, human hosts, and environment. Regarding the landscape constituents, particularly tree cover loss, forest fragmentation and other ecological factors strongly influence the mosquito vector populations [4–6].
Malaria control programmes are tasked with reducing the human infection rate using anti-malarial drugs, residual insecticides to kill the mosquito vectors, and the distribution of long-lasting insecticidal nets (LLINs) to decrease the human–mosquito contact rate [7]. However, most programmes have failed because of Plasmodium resistance to drugs, vector resistance to insecticides, absence of field malariology studies [8], and lack of integrated vector management [9]. The success of the latter depends on careful planning, sustainability, political support, and flexibility to reorient the programme based on robust field evidence. To build the basic control measures, it is necessary to improve knowledge of the mosquito population that will be the object of control. The peak-biting pattern is one of the crucial entomological metrics that needs to be addressed [10, 11] because ecological mechanisms leading to temporal variation within and between species are poorly understood [12] and represent the pivotal interface of human/vector contact.
Nyssorhynchus darlingi (formerly known as Anopheles (Nyssorhynchus) darlingi) is the primary vector of Plasmodium vivax and Plasmodium falciparum in the Amazon [13]. The peak biting activity and biting indices are some of the entomological metrics used to calculate the vectorial capacity of a mosquito vector population for monitoring the effectiveness of the control interventions. In vectorial capacity models, the biting rate (number of bites by host and time) assumes that biting is homogeneous and aggregated over time [14, 15]; therefore, close examination of the peak biting time is clearly warranted. Other entomological variables, such as mosquito abundance, density, larval habitat, climate data, seasonality, host feeding pattern, and infection rate, have been widely studied in relation to entomological control [16–18]. In addition, genetic variability appears to have an influence on multimodal blood-feeding behaviour of Ny. darlingi [19–23], and forest cover effect on the peak biting time of Ny. darlingi in areas in the Amazon River basin [24].
Malaria transmission can vary depending on both oscillations in the temperature and humidity [25] and density of mosquito vectors and Plasmodium parasites [16, 26, 27]. Thus, deforestation represents a major driver of malaria transmission dynamics because changes in land cover and land use are associated with microclimatic variation. In addition, environmental modifications cause changes in abiotic factors of the soil, such as pH, temperature, and solar radiation, shifting the biotic community interactions among microorganisms, plants, and animals, including mosquito diversity [28, 29]. In this new micro-ecosystem, larval habitats are the primary drivers of mosquito occurrence, increasing the abundance and fitness of dominant species [30–33]. Malaria risk can increase because of a higher probability of the rates of human–mosquito contact and boosted entomological inoculation [34, 35]. Changes in patterns of microclimatic conditions can affect mosquito longevity [36] and the extrinsic incubation period of the parasites [27]. Warmer temperatures can decrease the extrinsic incubation period of Plasmodium, favouring increased competence of the malaria vector population [37]. In addition, a high number of sporozoites in the female salivary glands can induce a higher biting rate, suggesting vector behaviour manipulation [38]. Other studies also show that factors such as microclimate, humidity or circadian cycle can influence mosquito behaviour in relation to blood intake and oviposition [39, 40]. Extending dry hours on various days, for example, can increase blood feeding and consequently vectorial transmission as the females attempt to survive dehydration [41]. Clearly, such a scenario could impact the biting time of parous and nulliparous females. Differences in the activity pattern of parous and nulliparous females were shown in a study conducted in the Brazilian Amazon in which a higher proportion of parous females were collected at 22:00–23:00 whereas nulliparous females were more abundant at 18:00–19:00 [22]. In contrast, a study of Anopheles arabiensis in southeastern Tanzania found no significant relationship between parity status and the mosquito biting time phenotype [42].
Forest cover proportion can be a relevant index for managing an effective malaria control strategy, because as a microclimate regulator it can be an indicative of mosquito biting behaviour. As an example, to attain more efficient vector control intervention and to steer the utilization and distribution of LLINs. The understanding of mosquito biting activity in general and infective and non-infective females in particular will be beneficial in comprehending the impact of using mosquito nets primarily due to the impracticality of expecting 12 h of protection. This is because during the early evening and early morning hours, people engage in activities such as cooking, eating, conversing with their families and friends, or praying outside the house [43].
Methods
Study sites and mosquito collection
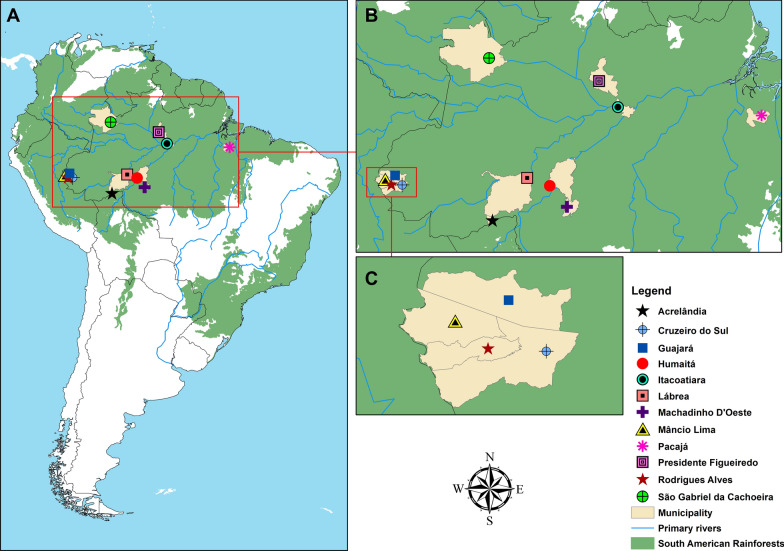
Females of the subfamily Anophelinae were collected in 80 houses in 12 municipalities in the Brazilian Amazon states of Acre, Amazonas, Pará and Rondônia states (Fig. 1; Table 1). The study locations were in rural settlements in areas with the mean annual malaria parasite incidence (Annual Parasite Incidence, API ≥ 30) of local P. vivax infections in the previous several months and during the period of field collections. The selection of field localities was also based on the level of forest cover, land use, and density of forest border as proxies of the presence of human and domestic animals (see [14] for additional details).
Fig. 1.
Field collection sites in 12 municipalities in the states of Acre, Amazonas, Pará and Rondônia, Brazil. A South America, B zoom-in of the studied area, C zoom-in of the municipalities of the Jurua River Valley
Table 1.
Total of Nyssorhynchus darlingi collected in human landing catches (HLC) from 18:00 to 0:00 in the peridomestic environment of 80 landscapes sites, including Plasmodium vivax and Plasmodium falciparum infection status of females, Amazon Basin, 2015–2017
| Municipality | State | Total (N) | Uninfected (n) | P. vivax-infected (n) | P. falciparum-infected (n) |
|---|---|---|---|---|---|
| Acrelândia | Acre | 173 | 173 | 0 | 0 |
| Cruzeiro do Sul | Acre | 331 | 325 | 6 | 0 |
| Guajará | Amazonas | 628 | 617 | 5 | 6 |
| Humaitá | Amazonas | 672 | 671 | 1 | 0 |
| Itacoatiara | Amazonas | 74 | 74 | 0 | 0 |
| Lábrea | Amazonas | 1395 | 1379 | 7 | 9 |
| Machadinho D’Oeste | Rondônia | 896 | 869 | 24 | 3 |
| Mâncio Lima | Acre | 616 | 611 | 1 | 4 |
| Pacajá | Pará | 17 | 17 | 0 | 0 |
| Presidente Figueiredo | Amazonas | 3639 | 3639 | 0 | 0 |
| Rodrigues Alves | Acre | 761 | 750 | 8 | 3 |
| São Gabriel da Cachoeira | Amazonas | 1343 | 1310 | 28 | 5 |
Female adult collections were conducted from January to November, during the wet, wet-dry transition, and dry seasons. Collections were outdoors in the peridomestic environment within ~ 5 m of each of 80 houses. Houses chosen for human landing catch (HLC) were at least 2.5 km apart and were positioned in the centre of a 1 km radius circle to avoid sampling more than one house within the same 3.14 km2 area. The selection of field localities was based on the level of forest cover, land use, and density of forest border as proxies of the presence of human and domestic animals. All field collections were carried out solely by ESB, GZL, LSMC, and MAMS. During the field collections, the researchers were wearing protective clothes, hats, and boots. Anopheline females were collected on the legs that were covered with thick black socks to avoid mosquito bites. A hand made 12-V battery powered manual aspirator was employed to take the mosquito females from the legs before they bite. Regional climates in the study region are classified as Tropical Rainforest (Af) and Tropical Monsoon (Am) (Köppen and Geiger Classification).
HLC collections were performed one night for each of 80 houses, from 18:00 to 0:00 (Table 1). The mosquito sampling effort was 480 h-collection, distributed as follows: Acrelândia 72 h, Cruzeiro do Sul 66 h, Humaitá, Itacoatiara, Lábrea, Machadinho D’Oeste, Mâncio Lima, Pacajá, Presidente Figueiredo 36 h each, São Gabriel da Cachoeira 42 h, and Guajará, Rodrigues Alves, 24 h each. Every hour, female mosquitoes were euthanized with ethyl acetate (C4H8O2) vapors in the field and stored in silica gel separated by date, location, house, and collection time. Specimens were morphologically identified to species level by MAMS, labelled and stored individually with silica gel at room temperature for subsequent analysis.
Mosquito processing
Genomic DNA was extracted from adult female Ny. darlingi using Qiagen DNeasy Blood & Tissue Kit (Hilden, Germany). All Ny. darlingi DNA samples were tested for Plasmodium spp. infection following [44], with DNA pools of up to five individuals containing equal amounts of gDNA. Mosquito samples with DNA concentrations of < 1.0 ng/µL or > 15 ng/µL were tested individually and not pooled. In instances where the species of Plasmodium could not be detected with the triplex assay, PCR amplification and agarose gel (2%) electrophoresis of PCR products was performed using primer pairs for P. vivax and P. falciparum [45]. Each PCR contained 1xPerfeCTa qPCR ToughMix, Uracil N-glycosylase (UNG), ROX (Quanta Biosciences, USA), 0.3 μM of each primer, ultrapure water, and 2 μL genomic DNA, with a total volume of 20 µL. Cycling conditions were as follows: 5 min UNG-activation hold at 45 °C and a denaturation step for 10 min at 95 °C, followed by 50 cycles of 95 °C denaturation for 15 s and 60 °C annealing/elongation for 1 min.
Landscape sites and forest cover estimation
Each landscape site (n = 80) was centred in a house with a local family. The HLC collections were accomplished in the peridomestic environment. The forest cover percentage (0–100%) within a 1-km radius (~ 3.14-km2) was calculated for each landscape site using QGIS v. 2.8. Forest cover values were categorized to depict three distinct deforestation scenarios: (1) degraded, < 30% forest cover, (2) intermediate, 30–70% forest cover, and (3) preserved, > 70% forest cover, as defined by [24].
Rationale of the study hypothesis
Nyssorhynchus darlingi females rest in vegetation nearby human dwellings during the day with subsequent flight to human residences at dusk (~ 18:00), when they bite humans or are captured by HLC. The study hypothesis is that the peak biting time of Ny. darlingi can vary according to forest cover (%) of a landscape site and status of infection of the mosquito (uninfected, P. vivax-infected, or P. falciparum-infected). The aims of the study were to test the association between forest cover percentage and (1) the peaking biting time, and (2) the number of non-infected and infected Ny. darlingi in the Brazilian Amazon.
Models for hypothesis testing
Assessment of the number of Ny. darlingi per status of infection and biting time in a gradient of forest cover (0–100%) was undertaken using the Huisman-Olff-Fresco (HOF) multi-model selection. The models’ equations and parameters are shown in Table 2.
Table 2.
Multi-model selection approach: models, formulas and parameters, responses, and expectations
| Model | Equations and parameters | Responses | Expectations |
|---|---|---|---|
| 1 | Uniform | Null hypothesis | |
| 2 | Linear | Abundance or infection correlates linearly with forest cover values | |
| 3 | Linear with Plateau | ||
| 4 | Unimodal | Abundance or infection has a unimodal correlation with forest cover values | |
| 5 | Asymmetric Unimodal | ||
| 6 | Bimodal | Abundance or infection has a bimodal correlation with forest cover values | |
| 7 | Asymmetric Bimodal |
Models were assumed having Poisson errors–Poisson regressions were worked out
#M is the maximum abundance or infection value which is within the positive integer set (1, 2, 3,…, n) for Poisson data. Model variables a–d and f are optimized in the process of model fitting [46]
Comparisons of total numbers of Ny. darlingi that were uninfected, P. vivax-infected, and P. falciparum-infected along this gradient were carried out at six different hour-long periods: 18:00–19:00; 19:00–20:00; 20:00–21:00; 21:00–22:00; 22:00–23:00; and 23:00–0:00. Regression curves were fitted to these data employing maximum likelihood estimates, the Akaike Information Criteria corrected for small samples (AICc), and bootstrap model checking. All analyses were run in R v. 4.3 (R Development, Core Team, Vienna, Austria). This approach has been successfully employed to test the intermediate disturbance hypothesis with mosquito community data in a gradient of forest cover in Panama [47]. Means and standard deviations per forest cover categories (< 30%, degraded; 30–70%, intermediate; and > 70%, preserved) were presented along with the regression curves.
Results
Study sites and anopheline collection
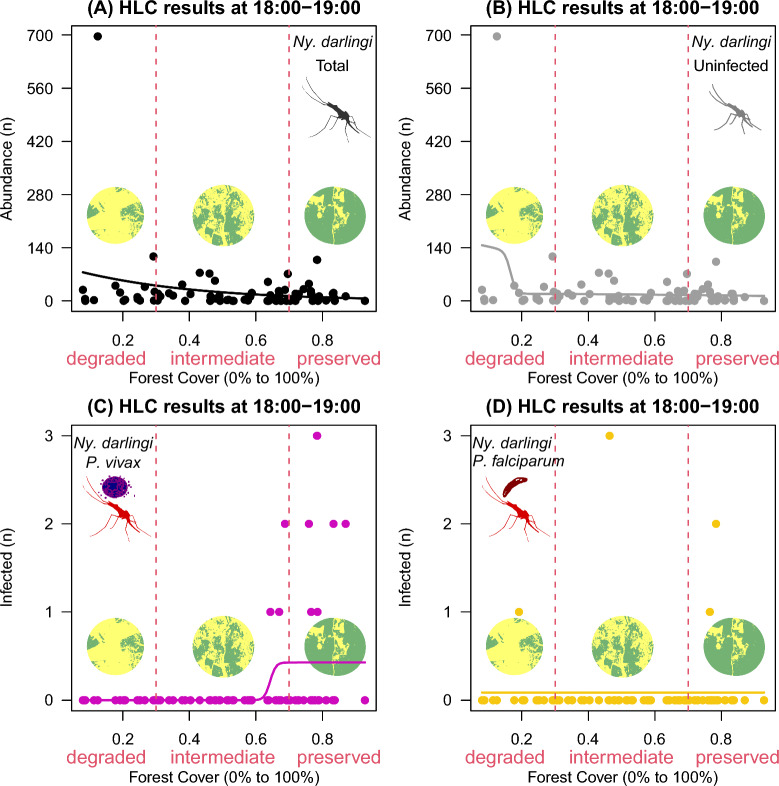
A total of 10,545 Ny. darlingi females were collected between 18:00 and 00:00 in 80 landscape sites in which forest cover varied from 8 to 93%. This total was composed of three groups of Ny. darlingi females: uninfected (10,435; 99%); infected with P. vivax (80; 0.75%); or with P. falciparum (30; 0.25%). In Figs. 2, 3, 4, 5, 6 and 7, the closed circles represent landscape-sites (n = 80) where outdoor HLC were performed, i.e., in panel A is shown the distribution of total abundance of Ny. darlingi collected per landscape-site, in panel B, the distribution of the uninfected fraction, in panel C, the P. vivax-infected and in panel D, the P. falciparum-infected. The black line represents the fitted curve from the best statistical model to the total Ny. darlingi abundance, the gray line is the curve from the best statistical model to the uninfected fraction, and analogously, magenta, and yellow curves represented the fitted statistical models to the infected P. vivax- and P. falciparum-Ny. darlingi fractions.
Fig. 2.
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch (HLC) at 18:00–19:00, according to infection status, along a gradient of forest cover (0–100%) across the 80 landscape sites
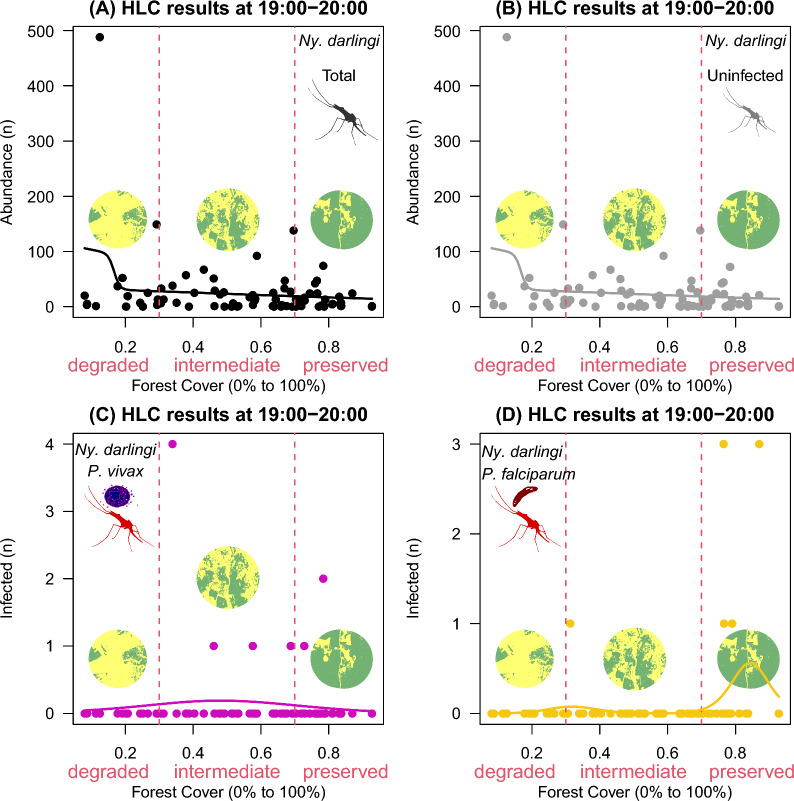
Fig. 3.
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch (HLC) at 19:00–20:00 according to infection status along a gradient of forest cover (0–100%) at landscape sites
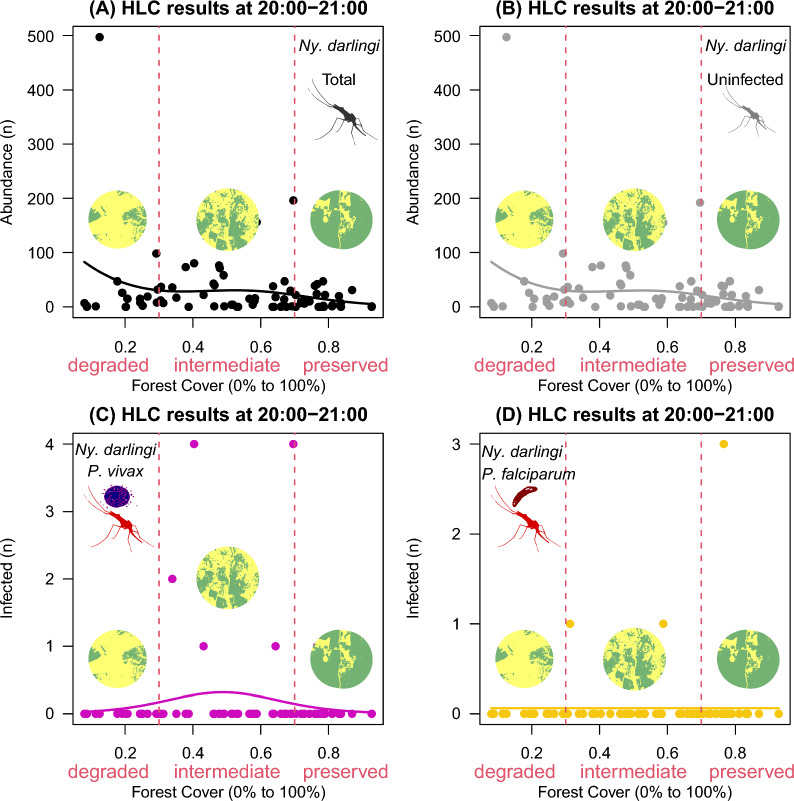
Fig. 4.
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch (HLC) at 20:00–21:00 according to infection status along a gradient of forest cover (0–100%) in landscape sites
Fig. 5.
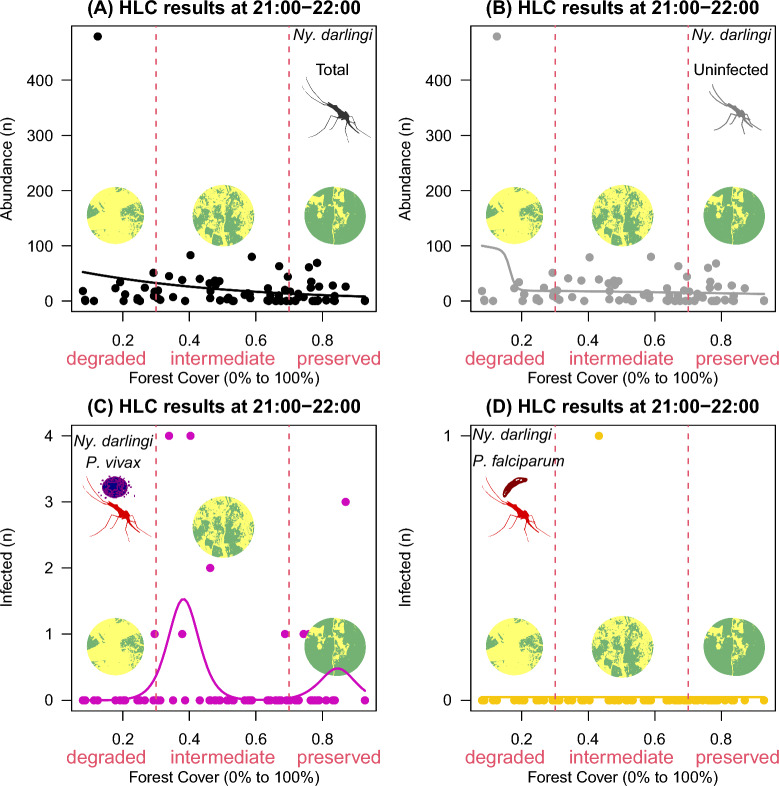
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch (HLC) at 21:00–22:00 according to infection status along a gradient of forest cover (0–100%) in landscape sites
Figure. 6.
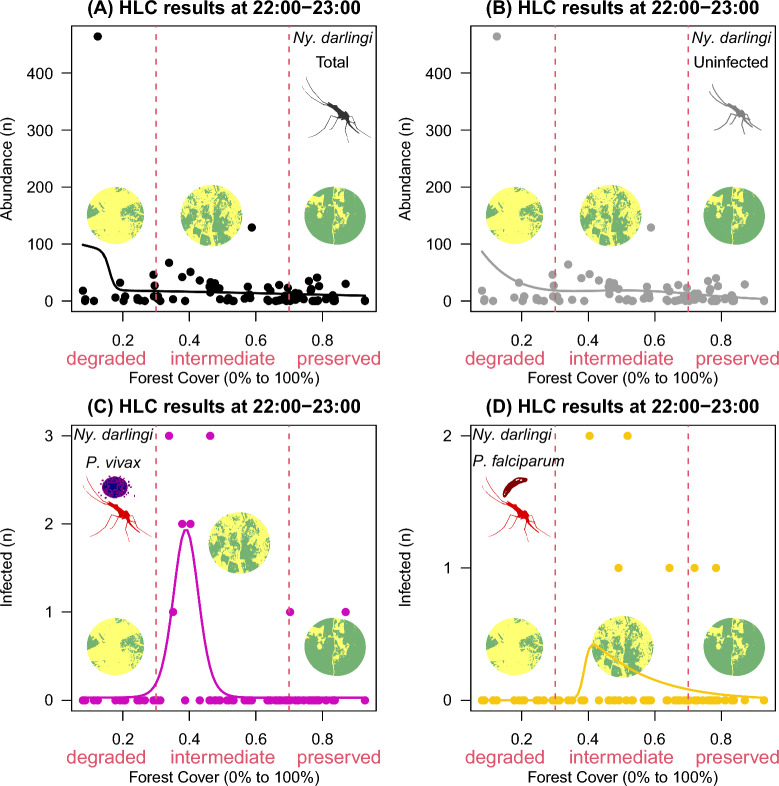
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch at 22:00–23:00 according to infection status along a gradient of forest cover (0–100%) in landscape sites
Fig. 7.
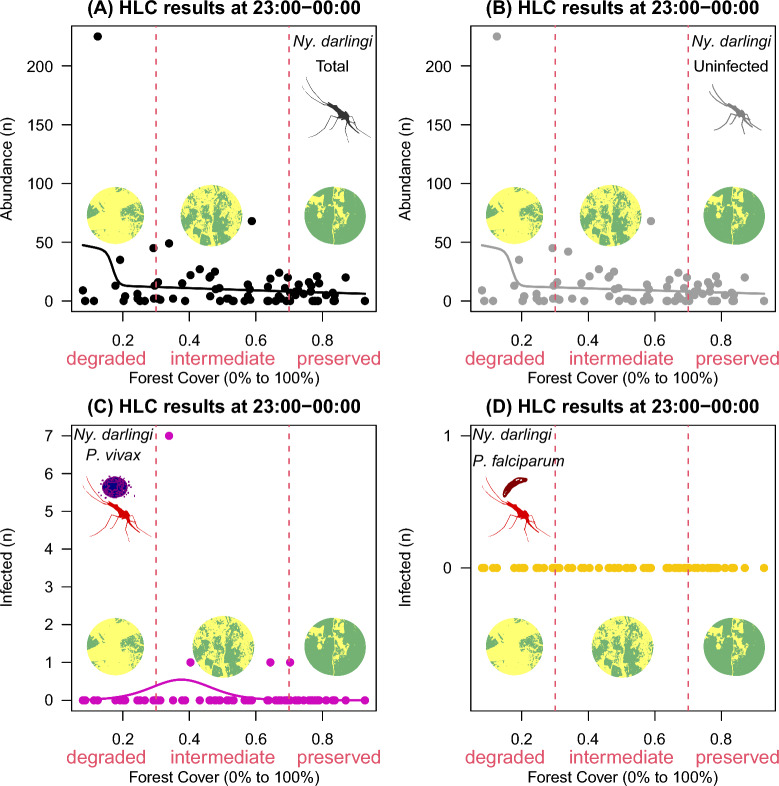
HOF multi-model selection scheme showing the fitted curve to Nyssorhynchus darlingi females sampled by human landing catch at 23:00–00:00 according to infection status along a gradient of forest cover (0–100%) in landscape sites
Overall, the uninfected female fraction had a different peak biting time compared with the infected counterparts. At 18:00–19:00, HLC yielded more infected and uninfected females (Fig. 2A) and more uninfected Ny. darlingi females in landscape sites with < 30% forest cover (62 ± 172 in degraded vs. 16 ± 22 at intermediate vs. 14 ± 21 in preserved; Fig. 2B). This difference was caused by a single observation, a collection made in one landscape-site in Presidente Figueiredo County, Amazonas state (Fig. 2B). If this observation is removed, the uninfected Ny. darlingi abundance was similar at any forest cover percentage. However, P. vivax-infected females were found in locations with > 70% forest cover (0 ± 0 in degraded vs. 0.1 ± 0.4 at intermediate vs. 0.4 ± 0.9 in preserved) (Fig. 2C), whereas P. falciparum-infected females were collected in small number in any forest cover percentage (Fig. 2D).
At 19:00–20:00, total females (Fig. 3A) peaked at degraded locations, P. vivax-infected Ny. darlingi females peaked at intermediate (Fig. 3C; 0 ± 0 in degraded vs. 0.2 ± 0.7 at intermediate vs. 0.1 ± 0.4 in preserved), and P. falciparum-infected at conserved (Fig. 3D; 0 ± 0 in degraded vs. 0.03 ± 0.16 at intermediate vs. 0.3 ± 0.8 in preserved) portions of the forest cover gradient, whereas the uninfected fraction showed a higher frequency at the degraded portion of this gradient ( 52 ± 122 in degraded vs. 26 ± 36 at intermediate vs. 13 ± 17 in preserved; Fig. 3B).
Nyssorhynchus darlingi females infected with P. vivax exhibited higher frequency levels at the intermediate portion (0 ± 0 in degraded vs. 0.3 ± 1 at intermediate vs. 0.04 ± 0.2 in preserved; Fig. 4C) of forest cover gradient at 20:00–21:00. No obvious pattern was seen for those infected with P. falciparum during this period (Fig. 4D). Total females (Fig. 4A) and uninfected females were found throughout the gradient, particularly in the degraded portion (49 ± 122 in degraded vs. 30 ± 42 at intermediate vs. 12 ± 14 in preserved; Fig. 4B).
A bimodal curve for intermediate and conserved portions of the forest cover gradient was seen for Ny. darlingi infected with P. vivax at 21:00–22:00 (0.06 ± 0.3 in degraded vs. 0.3 ± 1 at intermediate vs. 0.2 ± 0.7 in preserved; Fig. 5C). Total females (Fig. 5A) and uninfected females occurred in all forest cover levels (Fig. 5B), and P. falciparum infected females (Fig. 5D) showed no clear pattern.
Females infected with both P. vivax and P. falciparum showed specific responses at 22:00–23:00 peaking at the intermediate forest cover (0.0 ± 0.0, 0.0 ± 0.0 in degraded vs. 0.3 ± 0.8, 0.2 ± 0.5, at intermediate vs. 0.08 ± 0.3, 0.08 ± 0.3 in preserved; Fig. 6C, D). Total females (Fig. 6A) and uninfected females (Fig. 6B) had the same pattern as seen for the previous biting times (Figs. 2–5).
Lastly, P. vivax-infected females peaked at the intermediate forest cover in 23:00–00:00 (0 ± 0 in degraded vs. 0.2 ± 1 at intermediate vs. 0.04 ± 0.2 in preserved; Fig. 7C). No females infected with P. falciparum were found (Fig. 7D) and total (Fig. 7A) and uninfected females again showed no specific response to forest cover levels although there was an association with degraded landscape sites (Fig. 7B).
Discussion
Deforestation, forest fragmentation and the percentage of the forest cover in areas with endemic malaria transmission are key drivers of the density of species of Anophelinae that are Plasmodium vectors, increasing the likelihood of human exposure to infected mosquito bites, intensity of Plasmodium transmission, and spread of malaria [4, 48, 49]. Results of the current investigation, focused on the effects of tree cover loss on the biting behaviour of Ny. darlingi, showed that forest cover percentage can affect the peak biting time of this species across the Amazon River basin. The blood feeding behaviour of Ny. darlingi has been studied [12, 14, 50], and it is known that it has multimodal peaks [19, 21, 22], and usually exophilic biting behaviour [51], associated with environmental variables [52]. Recently, Oliveira et al. [24] determined that in rural settlements with high edge density and forest cover between 30 and 70%, the prevalence of Anophelinae mosquitoes is high, and the number of Ny. darlingi decreased across the 12-h collections from high abundance in the early evening to the lowest, in the early morning, between 03:00 and 06:00. In addition, the number of Plasmodium infected mosquitoes was significantly higher from midnight to 03:00 than from 18:00 to 21:00, 21:00 to 00:00, 03:00 to 06:00.
In the present study, the uninfected population of Ny. darlingi also demonstrated a different peak biting time compared to the infected populations. From 18:00 to 19:00, the HLC collections yielded a higher proportion of uninfected Ny. darlingi females in degraded areas with ~ 20% forest cover, whereas P. vivax-infected females were found in partially degraded areas with > 60% forest cover. Plasmodium vivax and P. falciparum-infected Ny. darlingi females peaked in areas with intermediate and high forest cover percentage, whereas the uninfected females showed higher frequency at the degraded portion of this gradient from 19:00 to 20:00.
Despite the evidence of the study’s findings, further investigations are needed to verify and quantify the influence of the percentage of forest cover and fragmentation on mosquito peak biting behaviour, and on the dynamics of Plasmodium transmission. Other variables that can influence mosquito community composition and life history, such as thermic amplitude of the soil surface, soil microbiome, and temperature of freshwater ecosystems in varied forest cover need to be investigated to understand any substantial impact they may exert on peak biting time of malaria vectors. Forest cover can influence the local microclimate by increasing the range of daytime temperature and humidity, variables essential to mosquito biting profiles. Recently, an investigation focusing on Anopheles farauti showed that temperature is an important predictor of the biting activity in Australia. Besides temperature, light intensity, humidity, collector, and season are important predictors. Despite being non-linear, the temperature had a positive effect on the female biting activity, whereas the impact of humidity was more complex [53]. Because deforestation decreases tree evapotranspiration, leading to changes in both the microclimate factors and seasonality of dry and wet seasons in the Amazon tropical rain forest [54], variation detected in the peak biting time of infected and non-infected females in a gradient of forest cover percentage may be linked to variation in temperature and humidity during the night. It is also important to consider that this study of Ny. darlingi biting activity was conducted across the wet, wet-dry transition, and dry seasons, thus the peak biting profile of infected and non-infected females, might also differ seasonally; furthermore, other infected/non-infected mosquito species might respond differently.
The study’s observation in rural settlements in the Amazon provide support for an association between the number of non-infected Ny. darlingi and low forest cover, whereas landscapes with > 75% of forest cover are associated with high numbers of infected mosquitoes. The latter finding can be explained by the high incidence of human malaria in the local inhabitants [24], likely because they have poor access to health facilities, delay in malaria diagnosis and anti-malarial treatment such as that observed in areas of frontier malaria [55]. In landscapes with > 75% forest cover, infected females peaked from 19:00 to 20:00 in the peridomestic environment when humans are generally still awake. Several factors may help to understand this peak biting time of infected females, for example, parous females may rest on the forest edge vegetation to digest blood and develop eggs, and the human dwellings are adjacent the forest edge, an easy source of a subsequent blood meal. It is also necessary to investigate whether differences in peak biting times have a genetic basis as demonstrated in Mâncio Lima, Acre state, Brazilian Amazon for Ny. darlingi [23]. In the northeastern Brazilian Amazon, a high frequency of parous Ny. darlingi females was collected from 20:00 to 22:00 whereas nulliparous females were more abundant from 18:00 to 19:00. Unfortunately, information on forest cover percentage and forest fragmentation is unavailable from this study in the district of Coração in the outskirts of Macapá, Amapá state, to compare potential associations among parity rate, peak biting time and forest cover [22].
Changes in biodiversity have been reported to increase malaria incidence [56–58]. Anthropogenic modifications in natural environments can cause changes in the richness and distribution of species [32], shifts in the circadian clock of a mosquito vector [59], and impact behaviours of a species: anthropophilic/zoophilic and exophagic/endophagic [51]. In areas with high mosquito diversity, diffuse competition for hosts can negatively impact blood feeding activity [60]. Therefore, the abundance of non-vector species can modify the peak temporal activity of Ny. darlingi, and diffuse competition can support this process.
Synergism among low forest cover, biodiversity loss, and changes in the local climate can shift the peak biting time of mosquitoes, as in species of the tribe Aedini and other groups in the Atlantic Forest [61]. Also, seasonality modulates the behaviour of the African malaria vector, An. arabiensis, shifting the biting preference from indoors to outdoors [62]. In the Amazon River basin, the density of malaria vectors depends on the annual seasonal cycle of rainfall. Both high precipitation and cooler temperatures during the rainy season and higher temperatures and lower precipitation in the dry season affect mosquito abundance [18, 63]. In this context, both environmental and landscape factors can influence on Ny. darlingi behaviour [17, 24]. In Blondin village in the Oyapock River, Amazonian French Guyana, Vezenegho et al. [64] found that a statistically significant higher percentage of Ny. darlingi was collected between 20:30 and 22:30 compared to those caught from 18:30 to 20:30 and from 05:00 to 07:00 at the long dry season. Despite the difference observed in Ny. darlingi peak biting time in the long dry season, there was no difference in the biting peaking time during the short rainy season.
In areas where Ny. darlingi is the dominant vector, the risk of malaria can be influenced by the control measures adopted, the vector species biting profile, and the plasticity in the blood feeding behaviour of the vector females [50, 65]. In addition, knowledge of the proportion of nulliparous or multiparous females can indicate the density of females that can carry Plasmodium parasites because of previous feeding on the blood of infective human [22]. This information can be a significant contribution to evaluate vector control strategies focused on decreasing malaria transmission.
The results of the current investigation revealed that areas with about 40% forest cover from 22:00 to 23:00, and localities with 83% forest cover from 19:00 to 20:00, had a higher proportion of P. vivax and P. falciparum infected Ny. darlingi. Conversely, a higher number of uninfected females was found in areas with less than 25% forest cover during all time slots examined. The link between percentage of forest cover and peak biting activity of infected and non-infected Ny. darlingi in the Amazon is supported by the results of the study. Additionally, the peak biting time of both P. vivax and P. falciparum infected and non-infected females varies depending on the time slot and forest cover percentage. These discoveries present an added hurdle for malaria control programmes because there is strong evidence that Ny. darlingi can transmit P. vivax and P. falciparum outdoors during the night, at different hours between 18:00 to midnight, depending on the percentage of forest cover.
Conclusions
In this study, the biting peak time of female Ny. darlingi infected and uninfected with Plasmodium was examined across a range of forest cover percentages in the peridomestic environment in rural settlements across Brazilian Amazon. Nyssorhynchus darlingi females can bite from 18:00 to midnight in all ranges of forest cover investigated. The proportion of P. vivax and P. falciparum infected and uninfected females fluctuated with the time of collection and forest cover percentage. The level of deforestation in the landscape affects the biting peak time of the infected females of Nyssorhynchus darlingi. Consequently, the temporal dynamic of infected females in the landscape is a key factor to looking for more effective control/elimination methods.
Acknowledgements
Authors are in debt to the Vector Malaria Control of the municipalities where field collections were conducted.
Abbreviation
- HLC
Human landing catch collection
Biographies
Leonardo Suveges Moreira Chaves
was an associate researcher at Departamento de Epidemiologia, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, SP, Brazil
Eduardo Sterlino Bergois
researcher at Instituto Pasteur, Secretaria de Estado da Saúde de São Paulo, Araraquara-SP, Brazil
Sara A. Bickersmith
works for the Wadsworth Center, New York State Department of Health, Albany, NY, USA
Gabriel Z. Laporta
is a professor at Graduate Program in Health Sciences, FMABC Medical School University Center, Santo André, SP, Brazil
Jan E. Conn
works for the Wadsworth Center, New York State Department of Health, Albany, NY, USA. JEC is a professor at Department of Biomedical Sciences, School of Public Health, State University of New York, Albany, NY, USA
Maria Anice Mureb Sallum
is a professor at Departamento de Epidemiologia, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, SP, Brazil
Author contributions
LSMC, JC, GZL, and MAMS designed the study. SAB conducted laboratory testing. GZL conducted the analysis. LSMC, GZL, and MAMS interpreted the data and results of analyses. GZL prepared the figures and tables. LSMC, GZL, JC, and MAMS wrote the manuscript. LSMC, MAMS, GZL and ESB conducted the field collections.
Funding
This investigation was supported by FAPESP grants 2014/26229-7 (MAMS), 2014/26855-5 (LSMC), 2014/09774-1 (GZL), CNPq grant 303382/2022-8 (MAMS), and National Institutes of Health, USA, grant 2R01AI110112-06A1 (JEC).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laurance WF, Cochrane MA, Bergen S, Fearnside PM, Delamônica P, Barber C, et al. The future of the Brazilian Amazon. Science. 2001;291:438–439. doi: 10.1126/science.291.5503.438. [DOI] [PubMed] [Google Scholar]
- 2.Kirby KR, Laurance WF, Albernaz AK, Schroth G, Fearnside PM, Bergen S, et al. The future of deforestation in the Brazilian Amazon. Futures. 2006;38:432–453. doi: 10.1016/j.futures.2005.07.011. [DOI] [Google Scholar]
- 3.Schneider M, Peres CA. Environmental costs of government-sponsored agrarian settlements in Brazilian Amazonia. PLoS ONE. 2015;10:e0134016. doi: 10.1371/journal.pone.0134016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaves LSM, Conn JE, López RVM, Sallum MAM. Abundance of impacted forest patches less than 5 km2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci Rep. 2018;8:7077. doi: 10.1038/s41598-018-25344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald AJ, Mordecai EA. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc Natl Acad Sci USA. 2019;116:22212–22218. doi: 10.1073/pnas.1905315116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin K, Bellinger M, Rana P. Anthropogenic forest loss and malaria prevalence: a comparative examination of the causes and disease consequences of deforestation in developing nations. AIMS Environ Sci. 2017;4:217–231. doi: 10.3934/environsci.2017.2.217. [DOI] [Google Scholar]
- 7.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria: global progress 2000–2015 and future challenges. Infect Dis Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kevin BJ. Malaria control by commodities without practical malariology. BMC Public Health. 2017;17:590. doi: 10.1186/s12889-017-4454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Handbook for integrated vector management (2012) Geneva: World Health Organization, http://whqlibdoc.who.int/publications/2012/9789241502801_eng.pdf. Accessed 1 October 2023
- 10.Harris AF, Matias-Arnéz A, Hill N. Biting time of Anopheles darlingi in the Bolivian Amazon and implications for control of malaria. Trans R Soc Trop Med Hyg. 2006;100:45–47. doi: 10.1016/j.trstmh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kabbale FG, Akol AM, Kaddu JB, Onapa AW. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District Uganda. Parasit Vectors. 2013;6:340. doi: 10.1186/1756-3305-6-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman RH, Lounibos LP, Nishimura N, Galardo AK, Galardo CD, Arruda ME. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar J. 2013;12:262. doi: 10.1186/1475-2875-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laporta GZ, Linton YM, Wilkerson RC, Bergo ES, Nagaki SS, Sant'Ana DC, et al. Malaria vectors in South America: current and future scenarios. Parasit Vectors. 2015;8:426. doi: 10.1186/s13071-015-1038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallum MAM, Conn JE, Bergo ES, Laporta GZ, Chaves LS, Bickersmith SA, et al. Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malar J. 2019;18:117. doi: 10.1186/s12936-019-2753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald G. Theory of the eradication of malaria. Bull World Health Organ. 1956;15:369. [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JM, Le Menach A, Pothin E, Eisele TP, Gething PW, Eckhoff PA, et al. Mapping multiple components of malaria risk for improved targeting of elimination interventions. Malar J. 2017;16:459. doi: 10.1186/s12936-017-2106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290. doi: 10.1186/s12936-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adde A, Dusfour I, Vezenegho S, Carinci R, Issaly J, Gaborit P, et al. Spatial and seasonal dynamics of Anopheles mosquitoes in Saint-Georges de l’Oyapock, French Guiana: influence of environmental factors. J Med Entomol. 2017;54:597–605. doi: 10.1093/jme/tjx031. [DOI] [PubMed] [Google Scholar]
- 19.Rosa-Freitas MG, Broomfield G, Priestman A, Milligan P, Momen H, Molyneux DH. Cuticular hydrocarbons, isoenzymes and behaviour of three populations of Anopheles darlingi from Brazil. J Am Mosq Control Assoc. 1992;8:357–366. [PubMed] [Google Scholar]
- 20.Tadei WP, dos Santos JMM, de Souza Costa WL, Scarpassa VM. Biologia de anofelinos amazônicos: XII. Ocorrência de espécies de Anopheles, dinâmica da transmissão e controle da malária na zona urbana de Ariquemes (Rondônia). Rev Inst Med Trop Sao Paulo. 1988;30:221–51. [DOI] [PubMed]
- 21.Gama RA, Santos RL, Santos Fd, Silva IM, Resende MC, Eiras ÁE. Periodicity of capture of the Anopheles darlingi Root (Diptera: Culicidae) in Porto Velho, Rondônia Brazil. Neotrop Entomol. 2009;38:677–682. doi: 10.1590/S1519-566X2009000500019. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa LMC, Souto RNP, dos Anjos Ferreira RM, Scarpassa VM. Behavioral patterns, parity rate and natural infection analysis in anopheline species involved in the transmission of malaria in the northeastern Brazilian Amazon region. Acta Trop. 2016;164:216–225. doi: 10.1016/j.actatropica.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez MVN, Alonso DP, Kadri SM, Rufalco-Moutinho P, Bernardes IAF, de Mello ACF, et al. Nyssorhynchus darlingi genome-wide studies related to microgeographic dispersion and blood-seeking behavior. Parasit Vectors. 2022;15:106. doi: 10.1186/s13071-022-05219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira TM, Laporta GZ, Bergo ES, Chaves LSM, Antunes JLF, Bickersmith SA, et al. Vector role and human biting activity of Anophelinae mosquitoes in different landscapes in the Brazilian Amazon. Parasit Vectors. 2021;14:1–13. doi: 10.1186/s13071-021-04725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong D, Wang X, Xu T, Zhou G, Wang Y, Lee M-C, Hartsel JA, Cui L, Zheng B, Yan G. Effects of microclimate condition changes due to land use and land cover changes on the survivorship of malaria vectors in China–Myanmar border region. PLoS ONE. 2016;11:e0155301. doi: 10.1371/journal.pone.0155301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parham PE, Michael E. Modeling the effects of weather and climate change on malaria transmission. Environ Health Perspect. 2010;118:620–626. doi: 10.1289/ehp.0901256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro LL, Whitehead SA, Thomas MB. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017;15:e2003489. doi: 10.1371/journal.pbio.2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos M, Alonso DP, Conn JE, Vinetz JM, Emerson KJ, Ribolla PEM. Genetic diversity of Nyssorhynchus (Anopheles) darlingi related to biting behavior in western Amazon. Parasit Vectors. 2019;12:1–9. doi: 10.1186/s13071-019-3498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira TMP, Sanabani SS, Sallum MAM. Bacterial diversity associated with the abdomens of naturally Plasmodium-infected and non-infected Nyssorhynchus darlingi. BMC Microbiol. 2020;20:1–8. doi: 10.1186/s12866-020-01861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiger DBM, Ritchie SA, Laurance SG. Mosquito communities and disease risk influenced by land use change and seasonality in the Australian tropics. Parasit Vectors. 2016;9:1–13. doi: 10.1186/s13071-016-1675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejmánková E, Grieco J, Achee N, Roberts DR. Ecology of larval habitats. Anopheles mosquitoes - New insights into malaria vectors. Intech. 2013 doi: 10.5772/55229. [DOI] [Google Scholar]
- 32.Chaves LSM, Bergo ES, Conn JE, Laporta GZ, Prist PR, Sallum MAM. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE. 2021;16:e0245087. doi: 10.1371/journal.pone.0245087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. 2018;26:101–110. doi: 10.1016/j.baae.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin KF. Brewing unequal exchanges in coffee: a qualitative investigation into the consequences of the Java Trade in Rural Uganda. J World-Systems R. 2017;23:326–352. doi: 10.5195/jwsr.2017.668. [DOI] [Google Scholar]
- 35.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission. Kenya Emerg Infect Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu V, Sallum M, Moore T, Lainhart W, Schlichting C, Conn J. Regional variation in life history traits and plastic responses to temperature of the major malaria vector Nyssorhynchus darlingi in Brazil. Sci Rep. 2019;9:5356. doi: 10.1038/s41598-019-41651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE. 2013;8:e79276. doi: 10.1371/journal.pone.0079276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends Parasitol. 2010;26:130–136. doi: 10.1016/j.pt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Fialho RF, Schall JJ. Thermal ecology of a malarial parasite and its insect vector: Consequences for the parasite’s transmission success. J Animal Ecol. 1995;64:553–562. doi: 10.2307/5799. [DOI] [Google Scholar]
- 40.Rowland M. Changes in the circadian flight activity of the mosquito Anopheles stephensi associated with insemination, blood-feeding, oviposition and nocturnal light intensity. Physiol Entomol. 1989;14:77–84. doi: 10.1111/j.1365-3032.1989.tb00939.x. [DOI] [Google Scholar]
- 41.Hagan RW, Didion EM, Rosselot AE, Holmes CJ, Siler SC, Rosendale AJ, et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-24893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milali MP, Sikulu-Lord MT, Govella NJ. Bites before and after bedtime can carry a high risk of human malaria infection. Malar J. 2017;16:91. doi: 10.1186/s12936-017-1740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14:e0217414. doi: 10.1371/journal.pone.0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bickersmith SA, Lainhart W, Moreno M, Chu VM, Vinetz JM, Conn JE. A sensitive, specific and reproducible real-time polymerase chain reaction method for detection of Plasmodium vivax and Plasmodium falciparum infection in field-collected anophelines. Mem Inst Oswaldo Cruz. 2015;110:573–576. doi: 10.1590/0074-02760150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laporta GZ, Burattini MN, Levy D, et al. Plasmodium falciparum in the southeastern Atlantic forest: a challenge to the bromeliad-malaria paradigm? Malar J. 2015;14:181. doi: 10.1186/s12936-015-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen F, Oksanen J. How to model species responses along ecological gradients - Huisman-Olff-Fresco models revisited. J Veg Sci. 2013;24:1108–1117. doi: 10.1111/jvs.12050. [DOI] [Google Scholar]
- 47.Loaiza JR, Dutari LC, Rovira JR, Sanjur OI, Laporta GZ, Pecor J, et al. Disturbance and mosquito diversity in the lowland tropical rainforest of central Panama. Sci Rep. 2017;7:7248. doi: 10.1038/s41598-017-07476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garg T. Ecosystems and human health: the local benefits of forest cover in Indonesia. J Environ Econ Manage. 2019;98:102271. doi: 10.1016/j.jeem.2019.102271. [DOI] [Google Scholar]
- 49.Kweka EJ, Kimaro EE, Munga S. Effect of deforestation and land use changes on mosquito productivity and development in Western Kenya Highlands: implication for malaria risk. Front Public Health. 2016;4:238. doi: 10.3389/fpubh.2016.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. doi: 10.4269/ajtmh.2006.74.3. [DOI] [PubMed] [Google Scholar]
- 51.Forattini OP. Exophilic behavior of Anopheles darlingi Root in a southern region of Brazil. Rev Saude Publica. 1987;21:291–304. doi: 10.1590/S0034-89101987000400002. [DOI] [PubMed] [Google Scholar]
- 52.Voorham J. Intra-population plasticity of Anopheles darlingi’s (Diptera, Culicidae) biting activity patterns in the state of Amapá. Brazil Rev Saude Publica. 2002;36:75–80. doi: 10.1590/S0034-89102002000100012. [DOI] [PubMed] [Google Scholar]
- 53.Chow WK, Beebe NW, Ambrose L, Pickering P, Cooper RD. Seasonal assessment on the effects of time of night, temperature and humidity on the biting profile of Anopheles farauti in north Queensland, Australia using a population naive to malaria vector control pressures. Malar J. 2023;22:85. doi: 10.1186/s12936-023-04495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laurance WF, Williamson GB. Positive feedbacks among forest fragmentation, drought, and climate change in the Amazon. Conserv Biol. 2001;15:1529–1535. doi: 10.1046/j.1523-1739.2001.01093.x. [DOI] [Google Scholar]
- 55.de Castro MC, Monte-Mór RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci USA. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson PT, Ostfeld RS, Keesing F. Frontiers in research on biodiversity and disease. Ecol Lett. 2015;18:1119–1133. doi: 10.1111/ele.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maliti DV, Marsden C, Main B, Govella N, Yamasaki Y, Collier T, et al. Investigating associations between biting time in the malaria vector Anopheles arabiensis Patton and single nucleotide polymorphisms in circadian clock genes: support for sub-structure among An. arabiensis in the Kilombero valley of Tanzania. Parasit Vectors. 2016;9:109. doi: 10.1186/s13071-016-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laporta GZ, de Prado PIKL, Kraenkel RA, Coutinho RM, Sallum MAM. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl Trop Dis. 2013;7:e2139. doi: 10.1371/journal.pntd.0002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos EB, Favretto MA, Müller GA. When and what time? On the seasonal and daily patterns of mosquitoes (Diptera: Culicidae) in an Atlantic Forest remnant from Southern Brazil. Austral Entomol. 2020;59:337–344. doi: 10.1111/aen.12454. [DOI] [Google Scholar]
- 62.Ngowo HS, Kaindoa EW, Matthiopoulos J, Ferguson HM, Okumu FO. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2:102. doi: 10.12688/wellcomeopenres.12928.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koenraadt CJ, Githeko AK, Takken W. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village [published correction appears in Acta Trop. 2004;90:301–2] Acta Trop. 2004;90:141–153. doi: 10.1016/j.actatropica.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Vezenegho SB, Adde A, Pommier de Santi V, et al. High malaria transmission in a forested malaria focus in French Guiana: how can exophagic Anopheles darlingi thwart vector control and prevention measures? Mem Inst Oswaldo Cruz. 2016;111:561–569. doi: 10.1590/0074-02760160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagaki SS, Chaves LSM, López RVM, Bergo ES, Laporta GZ, Conn JE, et al. Host feeding patterns of Nyssorhynchus darlingi (Diptera: Culicidae) in the Brazilian Amazon. Acta Trop. 2021;213:105751. doi: 10.1016/j.actatropica.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.