Fig. 2.
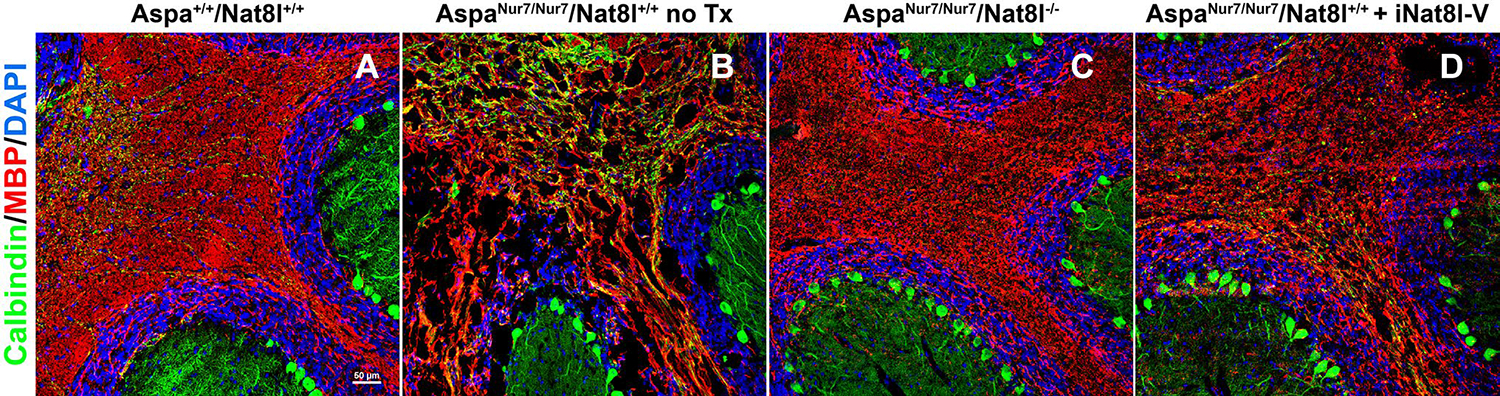
Cerebellar vacuolation and dysmyellnation in AspaNur7/Nur7 mice is prevented by homozygous constitutive Nat8l knockout, and substantially diminished in severity by neonatal brain Nat8l knockdown. Cryostat sections through cerebellum were prepared from brains of 2 months old wild-type (Aspa+/+/Nat8l+/+) (a), a 2 months old untreated A spaNur7/Nur7/Nat8l+/+ mouse (b), a 2 months old Aspa Nur7/Nur7/Nat8l−/− mouse (c), and a 2 months old A spaNur7/Nur7/Nat8l+/+ mouse that had been given an intracerebroventricular AAV carrying an Nat8l short hairpin inhibitory RNA (iNat8l-V) on postnatal day 1 (d). The sections were immunostained for myelin basic protein (MBP) and calbindin, counterstained with DAPI, and viewed by laser scanning confocal microscopy. Cerebellar vacuolation and dysmyelination were prominent in the untreated AspaNur7/Nur7 mouse, but these abnormalities were prevented by homozygous constitutive Nat8l knockout, and diminished in severity by Nat8l knockdown. Scale bar = 50μ m in all panels
