Abstract
Quadrigeminal cysts represent intracranial cystic accumulations of cerebrospinal fluid within the arachnoid mater at the level of the quadrigeminal cistern. Quadrigeminal cysts are rare in cats, with only one previous report in the veterinary literature. A 4-year-old, male-neutered Persian cat was presented with a 1-year duration of initially episodic, but later progressive, obtundation and collapse. Magnetic resonance imaging of the brain revealed a quadrigeminal cyst with marked compression of the adjacent neural structures, cerebellar herniation and obstructive hydrocephalus. Cystoperitoneal shunt placement was performed after the cat became refractory to medical therapy and this resulted in return of normal neurological status. The improvement in the neurological deficits following placement of a cystoperitoneal shunt in this case appeared to be correlated with resolution of the secondary effects (in particular the obstructive hydrocephalus) rather than resolution of the quadrigeminal cyst. Cystoperitoneal shunt placement was an effective long-term treatment option for the management of the quadrigeminal cyst in this cat.
A quadrigeminal cyst arises from an anomaly of the arachnoid membrane, in which duplication or splitting of the arachnoid membrane occurs within the quadrigeminal cistern during embryonic development. This results in collection of cerebrospinal fluid (CSF) within the arachnoid membrane, forming a cystic structure. 1–3 Controversy surrounds the clinical significance of quadrigeminal cysts in both dogs and humans, with many quadrigeminal cysts detected as apparently incidental findings during advanced brain imaging studies. 4–8 Neurological deficits may become evident if gradual expansion of the quadrigeminal cyst results in increased intracranial pressure, compression of adjacent neural structures or obstruction of CSF outflow pathways. 9–12 Quadrigeminal cysts in dogs are uncommon with a recent report suggesting a prevalence of 0.7% in dogs referred for investigation of intracranial disease. 8 Quadrigeminal cysts appear to be rare in cats with only one previous case report, 13 although the true prevalence in cats has yet to be determined.
A 4-year-old, male-neutered Persian cat with a 12-month history of episodic collapse and altered mentation was presented to the small animal neurology service at the University of Glasgow Small Animal Hospital. The episodes initially occurred every 3 months but in the last quarter, the cat had collapsed on six occasions. The cat presented as an emergency during a severe episode. Physical examination findings, other than those related to the nervous system, were normal. On neurological examination, the cat was severely obtunded with non-ambulatory tetraparesis. Hopping responses and tactile placing reactions were absent in all limbs. Cranial nerve examination revealed blindness with absent menace responses, intact pupillary light reflexes and bilateral, positional, ventro-lateral strabismus. Normal physiological nystagmus was absent in both eyes. Mild discomfort was evident on palpation of the cranial cervical spine. The neuroanatomical localisation was consistent with an extensive or multifocal central nervous system (CNS) lesion involving the brain and possibly the cervical spinal cord. Routine clinical assessment revealed no underlying biochemical or infectious cause for the abnormalities. Haematology revealed only lymph-openia (0.42×109/l; reference 1.5−7×109/l); serum biochemistry was unremarkable. Urinalysis, abd-ominal ultrasound, and chest radiographs were unremarkable. Analysis of his blood thiamine concentration and thiamine levels of his food were within a normal range. The serum lead level was very low and α-1-acid glycoprotein was mildly increased (1.39 g/l; reference 0.02–1 g/l).
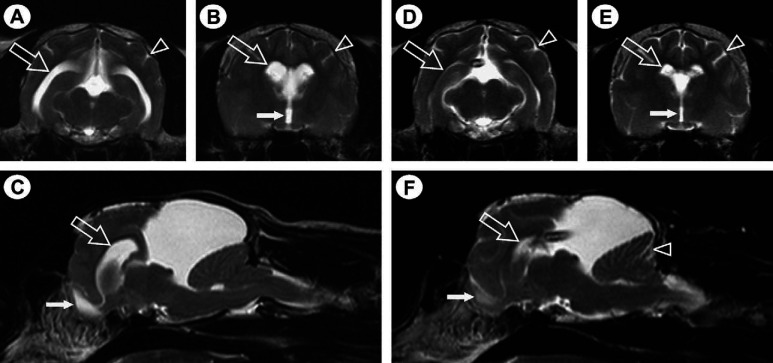
Magnetic resonance (MR) imaging of the brain was performed using a 1.5-Tesla MRI system (Gyroscan ACS NT, Philips Medical Systems, Eindhoven, the Netherlands) and an extremity coil. A large hyperintense, cystic lesion was present dorsal to the colliculi on sagittal T2-weighted MR images ( Fig 1 C). The signal intensity of the content of this lesion was identical to CSF. The lesion was hypointense on T1-weighted images with no contrast enhancement on administration of gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, UK). The cerebellum was compressed caudo-ventrally, with displacement of the caudal aspect of the cerebellum through the foramen magnum into the cervical spinal canal, and symmetrical obstructive hydrocephalus was present ( Fig 1 A–C). The MR imaging findings were consistent with a diagnosis of a quadrigeminal cyst with secondary obstructive hydrocephalus and cerebellar herniation.
Fig 1.
T2-weighted transverse (A, B, D and E) and para-sagittal (C and F) MR images, demonstrating a large hyperintense, cystic lesion dorsal to the colliculi (C and E). Images A–C are prior to cystoperitoneal shunt placement and images D–F are post cystoperitoneal shunt placement. The MR imaging appearance is consistent with a quadrigeminal cyst compressing the cerebellum and brain stem ventrally and the cerebral hemispheres rostrally. Evidence of obstructive hydrocephalus resulting in increased intracranial pressure is evident as enlargement of the lateral (open arrows in A–C) and third (filled arrow in B) ventricles, dilation of the olfactory bulb cavity (filled arrow in C), attenuation of the hyperintense CSF signal within the cerebral sulci (arrowhead in A and B) and between the folia of the cerebellum and displacement of the caudal aspect of the cerebellum into the cervical spinal canal. Resolution of the features suggestive of secondary obstructive hydrocephalus is evident following placement of a cystoperitoneal shunt (D–F), with the shunt-tip visible in images D and F.
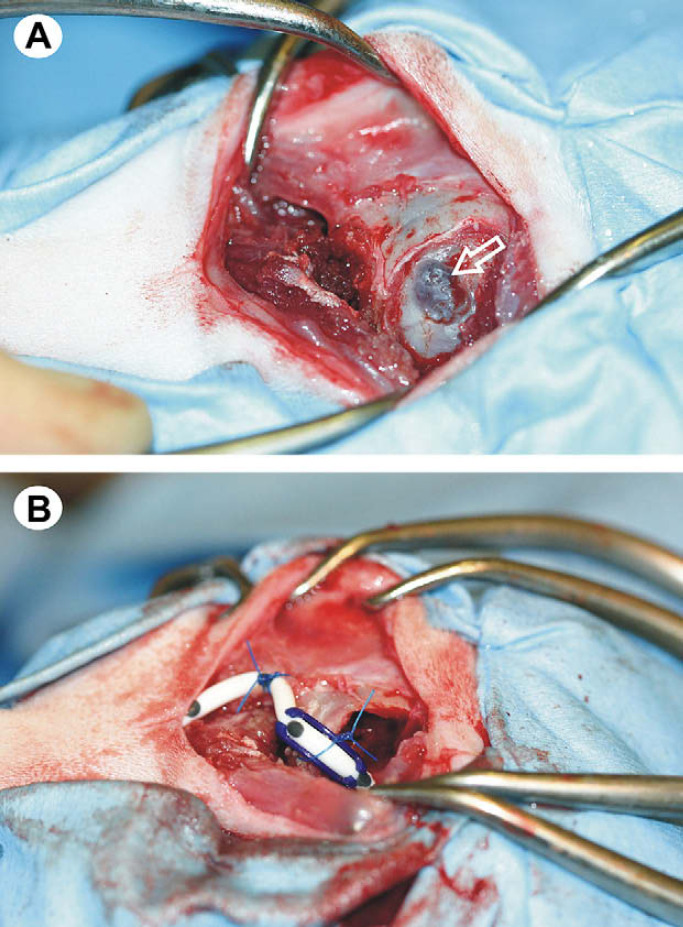
The volume of the lateral ventricles was calculated from the MR images using the technique described by Logan et al 14 and was found to be 1491 mm3. Medical therapy comprising prednisolone at 0.5 mg/kg q12 h orally (Prednicare; Animalcare, York, UK) and frusemide at 0.5 mg/kg q8 h orally (Frusecare; Animalcare, York, UK) was started. Initially, the cat's neurological status improved, but 7 days later the cat deteriorated and represented with non-ambulatory tetraparesis and decreased level of consciousness. As the response to medical therapy had been poor, surgical intervention was indicated. A cystoperitoneal shunt was placed using a modification of the technique described in the dog by Dewey et al. 15 The rostral extension of the quadrigeminal cyst in the cat meant that a smaller craniectomy defect could be created, allowing the transverse venous sinus to be spared ( Fig 2 ). Prednisolone therapy was re-started 24 h postoperatively at 0.5 mg/kg q24 h orally for 7 days, followed by a decrease to 0.5 mg/kg q48 h orally for the last 7 days. Two days following surgery the cat was normal with no neurological deficits; it has remained neurologically normal. The cat has remained neurologically normal for 10 months following the surgery. Repeated MR imaging of the brain 4 months after surgery revealed some reduction in the size of the quadrigeminal cyst ( Fig 1 F), but more significantly the obstructive hydrocephalus had resolved ( Fig 1 D–F). Calculation of lateral ventricle volume revealed a reduction from 1491 mm3 pre-surgical to 138 mm3 post-surgical.
Fig 2.
Intra-operative images, demonstrating cystoperitoneal shunt placement. In both images, rostral is to the right and caudal to the left. The apposed dura and cyst wall are visible within the craniectomy defect (open arrow in A). Successful placement of the cystoperitoneal shunt, with fixation of the shunt tubing to the periosteum of the occipital bone (B).
This is the second reported case of a quadrigeminal cyst in a cat. 13 There are insufficient feline cases to draw conclusions regarding breed or sex disposition but it is interesting to note that both cases were male Persian (brachycephalic) cats. Quadrigeminal cysts within the canine population are over-represented in male, brachycephalic, small-breed dogs. 4–8,16 Hydrocephalus has been noted in dogs with quadrigeminal cysts, but this has been considered a result of the brachycephalic breed disposition for dilated lateral ventricles rather than a pathological event. 5 However, in human patients, obstructive hydrocephalus is a frequent feature of the disease due to compression of the mesencephalic aqueduct by the enlarging quadrigeminal cyst.9,11 The proposed mechanism for cyst enlargement includes a ball valve mechanism allowing unidirectional CSF entry into the cyst, 12 fluid secretion by the ciliated epithelium of the cyst wall 10 and intra-cystic haemorrhage (although few reports have demonstrated evidence of intra-cystic haemorrhage in the absence of trauma). 17
The resolution of the neurological deficits following placement of a cystoperitoneal shunt in our case appeared to largely be correlated with resolution of the secondary obstructive hydrocephalus rather than resolution of the quadrigeminal cyst itself. Cystoperitoneal shunt placement was an acceptable and successful method for managing the quadrigeminal cyst in this cat and could be considered in others that are unresponsive to medical therapy. Long-term studies are required to assess the morbidity of this procedure compared to other surgical and medical managements.
References
- 1.Starkman S.P., Brown T.C., Linell E.A. Cerebral arachnoid cyst, J Neuropathol Exp Neurol 17, 1958, 484–500. [DOI] [PubMed] [Google Scholar]
- 2.Rengachary S.S., Watanabe I., Brackett C.E. Pathogenesis of intracranial arachnoid cysts, Surg Neurol 9, 1978, 139–144. [PubMed] [Google Scholar]
- 3.Rengachary S.S., Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts, J Neuropathol Exp Neurol 40, 1981, 61–83. [PubMed] [Google Scholar]
- 4.Vernau K.M., Kortz G.D., Koblik P.D., LeCouteur R.A., Bailey C.S., Pedroia V. Magnetic resonance imaging and computed tomography characteristics of intracranial intra-arachnoid cysts in 6 dogs, Vet Radiol Ultrasound 38, 1997, 171–176. [DOI] [PubMed] [Google Scholar]
- 5.Saito M., Olby N.J., Spaulding K. Identification of arachnoid cysts in the quadrigeminal cistern using ultrasonography, Vet Radiol Ultrasound 42, 2001, 435–439. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa M., Kanayama K., Sakai T. Quadrigeminal cisterna arachnoid cyst diagnosed by MRI in five dogs, Aust Vet J 81, 2003, 340–343. [DOI] [PubMed] [Google Scholar]
- 7.Duque C., Parent J., Brisson B., Da Costa C., Poma R. Intracranial arachnoid cysts: are they clinically significant?, J Vet Intern Med 19, 2005, 772–774. [DOI] [PubMed] [Google Scholar]
- 8.Matiasek L.A., Platt S.R.P., Shaw S., Dennis R. Clinical and magnetic resonance imaging characteristics of quadrigeminal cysts in dogs, J Vet Intern Med 21, 2007, 1021–1026. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T., Kuratomi A., Kuramoto S. Arachnoid cyst of the quadrigeminal cistern, Surg Neurol 14, 1980, 267–273. [PubMed] [Google Scholar]
- 10.Go K.G., Houthoff H.J., Blaauw E.H., Havinga P., Hartsuiker J. Arachnoid cyst of the silvian fissure. Evidence of fluid secretion, J Neurosurg 60, 1984, 803–813. [DOI] [PubMed] [Google Scholar]
- 11.Nishida K., Nakagawa Y., Fujimato N., Matsumoto K. A case of quadrigeminal cistern arachnoid cyst associated with hydrocephalus, No Shinkei Geka 16, 1988, 857–861. [PubMed] [Google Scholar]
- 12.Santamarta D., Aguas J., Ferrer E. The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism, Minim Invasive Neurosurg 38, 1995, 133–137. [DOI] [PubMed] [Google Scholar]
- 13.Milner R.J., Engela J., Kirberger R.M. Arachnoid cyst in cerebellar pontine area of a cat – diagnosis by magnetic resonance imaging, Vet Radiol Ultrasound 37, 1996, 34–36. [Google Scholar]
- 14.Logan A, Innocent G, Carrera I, Dennis R, Penderis J. Quantification of brain and cerebral ventricle volume using magnetic resonance imaging in the normal and abnormal canine brain. Proceedings of the 51st Annual Congress of the British Small Animal Veterinary Association; 2008. Apr 3–6; Birmingham, England. 2008: 457.
- 15.Dewey C., Krotscheck U., Bailey K.S., Marino D.J. Craniotomy with cystoperitoneal shunting for treatment of intracranial arachnoid cysts in dogs, Vet Surg 36, 2007, 416–422. [DOI] [PubMed] [Google Scholar]
- 16.Platt S.R. What is your diagnosis?, J Small Anim Pract 43, 2002, 425. [PubMed] [Google Scholar]
- 17.Vernau K.M., LeCouteur R.A., Sturges B.K., et al. Intracranial intra-arachnoid cyst with intracystic haemorrhage in two dogs, Vet Radiol Ultrasound 43, 2002, 449–454. [DOI] [PubMed] [Google Scholar]