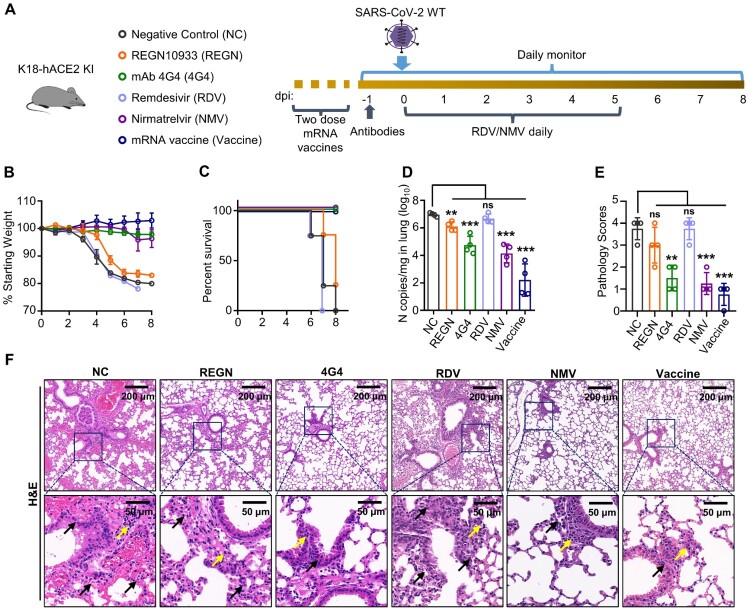
Figure 8.
Evaluation of anti-SARS-CoV-2 drug by K18-hACE2 KI mouse model. (A) Schematic of experimental design for anti-SARS-CoV-2 neutralizing antibody, mRNA vaccine, and drug treatment. Twelve-week-old K18-hACE2 KI mice were injected with 10 mg/kg REGN10933 or 4G4 at – 1 dpi, or treated with 25 mg/kg Remdesivir (RDV) daily by intraperitoneally injection from 0 to 5 dpi, or treated with 300 mg/kg Nirmatrelvir (NMV) daily by oral gavage from 0 to 5 dpi. For mRNA vaccine, mice received the first dose of mRNA vaccine intramuscularly 6 weeks prior to infection, followed by the second dose 3 weeks before infection. All groups K18-hACE2 KI mice were infected i.n. with 2.5 × 102 PFU SARS-CoV-2 WT viruses at 0 dpi. Mice that lost over 20% of their starting body weight were euthanized. Infected mice were monitored and evaluated at the indicated time for (B) body weight changes (n = 4), (C) survival (n = 4) and (D) viral N gene copy numbers at experimental endpoint. (E) Pathology scores. (F) The lungs of K18-hACE2 KI mice collected at experimental endpoint were subjected to H&E staining and histology scoring. The scale bar is shown in each section. The black arrow indicates the lung tissue damage, the yellow arrow indicates the infiltration of monocytes. One-way ANOVA with Tukey’s multiple comparison test was used. ***, P < 0.001; **, P < 0.01; ns, not significant, P > 0.05. Error bars represent the means with SEMs in (B) and the means with SDs elsewhere.