Figure 1.
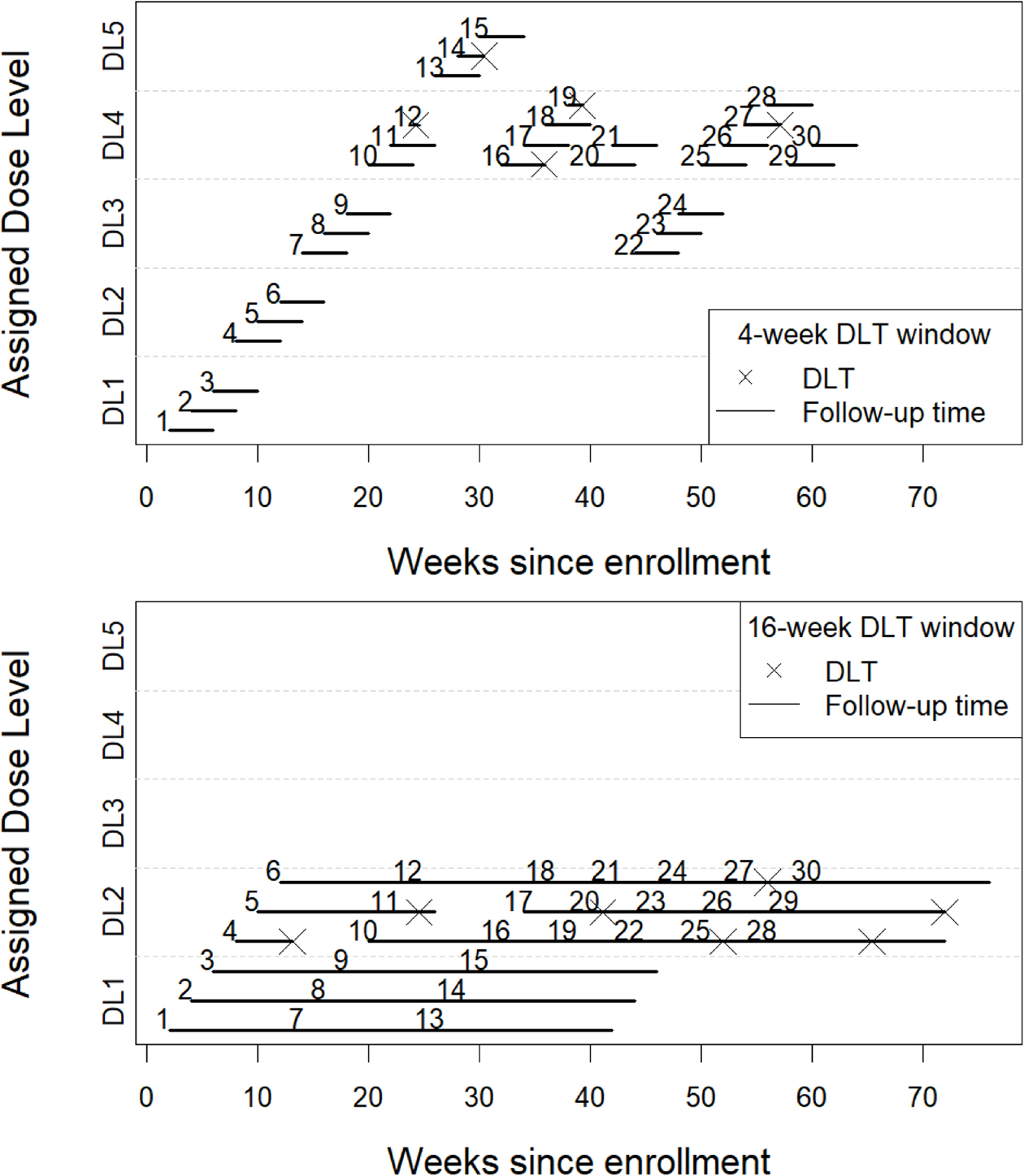
Trial data from an illustrative simulated example using a TITE-CRM with a 16-week observation window for toxicity (plot on top) and 4-week observation window (plot on bottom), a total sample size of 30 patients, an accrual rate of 2 patients per month. DL: dose level, DLT: Dose-limiting toxicity
