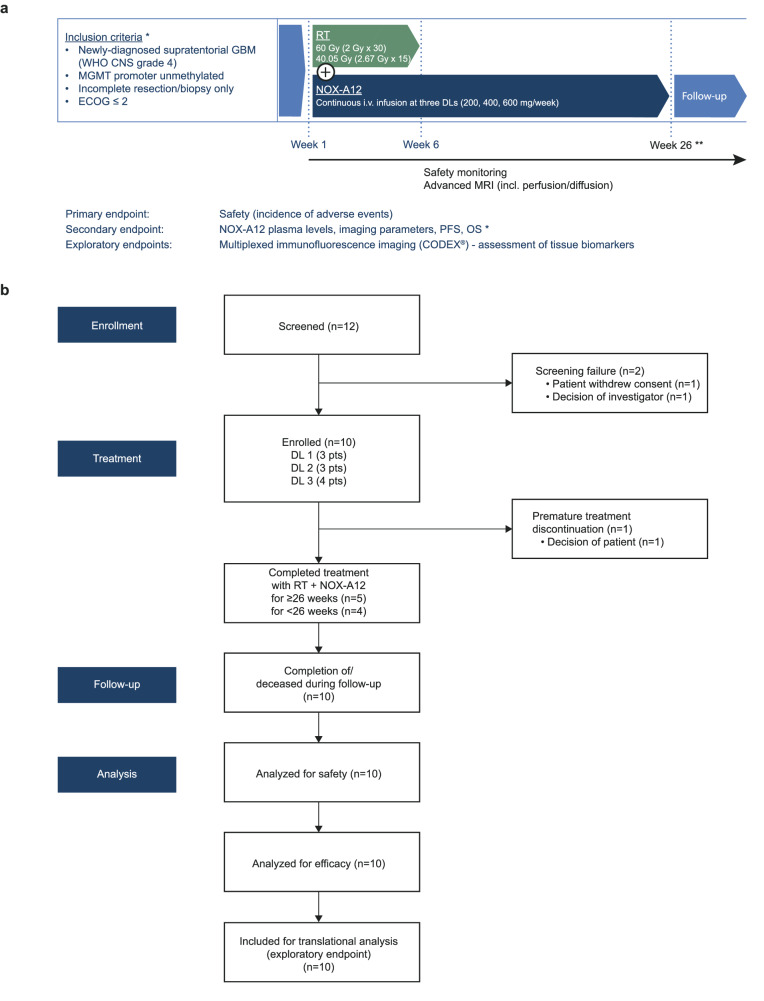
Fig. 1. Study outline of the GLORIA trial.
a Graphical overview of the study. GLORIA is a multicentric phase I/II trial conducted to assess the safety and efficacy of RT combined with escalating DLs of continuous i.v. treatment with NOX-A12 in newly diagnosed, incompletely resected, or biopsied GBM (CNS WHO grade 4) lacking MGMT promoter methylation (n = 10). *A complete and more detailed list of eligibility criteria and outcome measures is provided under ClinicalTrials.gov Identifier: NCT04121455. **End of treatment: 26 weeks as per protocol; treatment continuation beyond 26 weeks per investigator’s choice if the patient has clear clinical benefit. b Flow chart of the study. CODEX® CO-Detection by indEXing, DL dose level, ECOG Eastern Cooperative Oncology Group performance score, GBM glioblastoma, MGMT O6-methylguanine DNA methyltransferase, MRI magnetic resonance imaging, NOX-A12 olaptesed pegol, OS overall survival, PFS progression-free survival, RT radiotherapy.