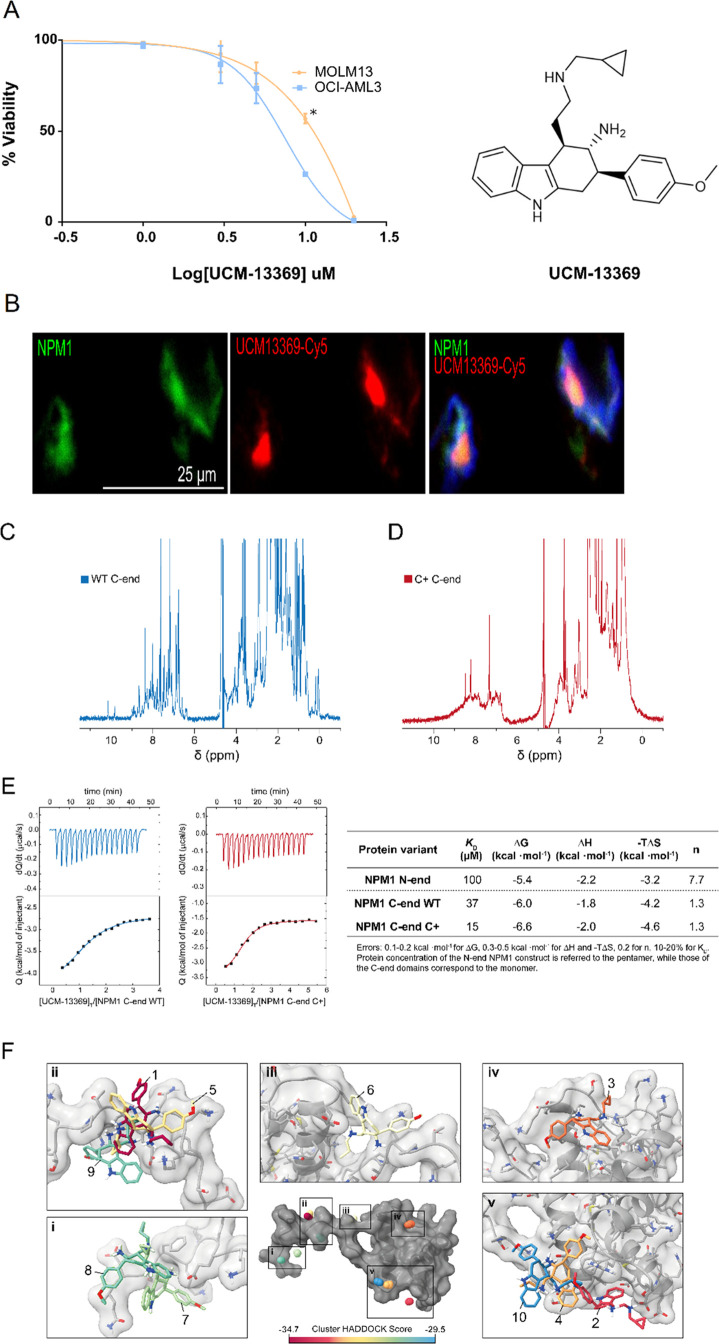
Figure 3.
Compound UCM-13369 targets NPM1 protein, binding mainly through the C-end domain of the C+ mutant form. (A) Dose–response curves of UCM-13369 in AML cell lines MOLM13 (NPM1 WT) and OCI-AML3 (NPM1 C+). (B) Confocal microscopy images with staining of NPM1 (Alexa Fluor-488, green), UCM-13369-Cy5 (red), and nuclei (DAPI, blue) in OCI-AML3 cells. (C,D) 1D 1H NMR spectra of the C-end domain of NPM1 WT (C) and C+ (D). (E) ITC thermograms and binding isotherms of the interaction of UCM-13369 with the WT (blue) and C+ mutant (red) forms of NPM1. ITC-derived thermodynamic parameters of UCM-13369–NPM1 interaction. Gibbs free energy (ΔG), enthalpy (ΔH), entropic term (-TΔS), equilibrium dissociation constant (KD), and binding stoichiometry (n). (F) Visualization of UCM-13369 binding interfaces to NPM1 C-end domain. The centroids of UCM-13369 in the four lowest-energy structures of each 10 clusters are plotted as balls colored according to the HADDOCK score, as indicated in the color legend. The structure of UCM-13369 of the top-ranked structure of each cluster is represented in the insets (i-v).