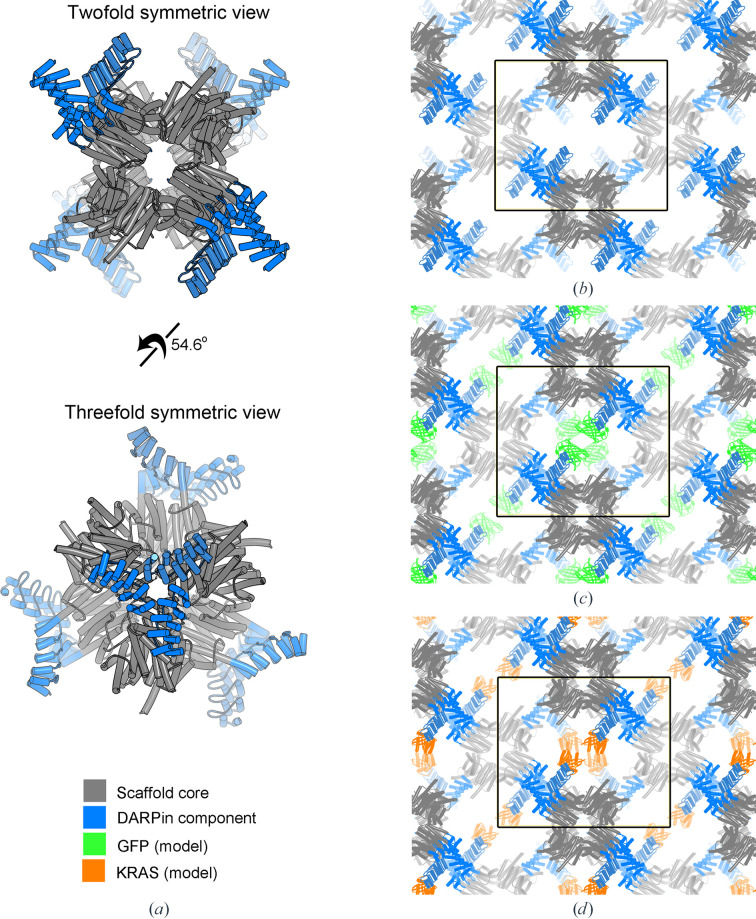
Figure 3.
Structure of the DARP3 scaffold and its crystal packing. (a) Views of the fully assembled 24-subunit DARP3 scaffold along the twofold (top) and threefold (bottom) axes of symmetry. The crystal structure closely resembles the structure of the KRAS-binding DARPin scaffold (Castells-Graells et al., 2023 ▸). The asymmetric unit consists of a trimer of the DARPin-fused component and the unfused native cage component. (b) Crystal packing of the DARP3 assembly. A large solvent channel is present between four copies of the DARP3 scaffold. One DARPin from each scaffold points into the cavity, allowing cargo binding at one of the available DARPins. (c) A model illustrating that GFP molecules could theoretically fit into the solvent channel without steric clashes when bound to one of the three DARPin modules. (d) A model illustrating that the same is true for KRAS. The black outline denotes the unit cell.