Figure 1.
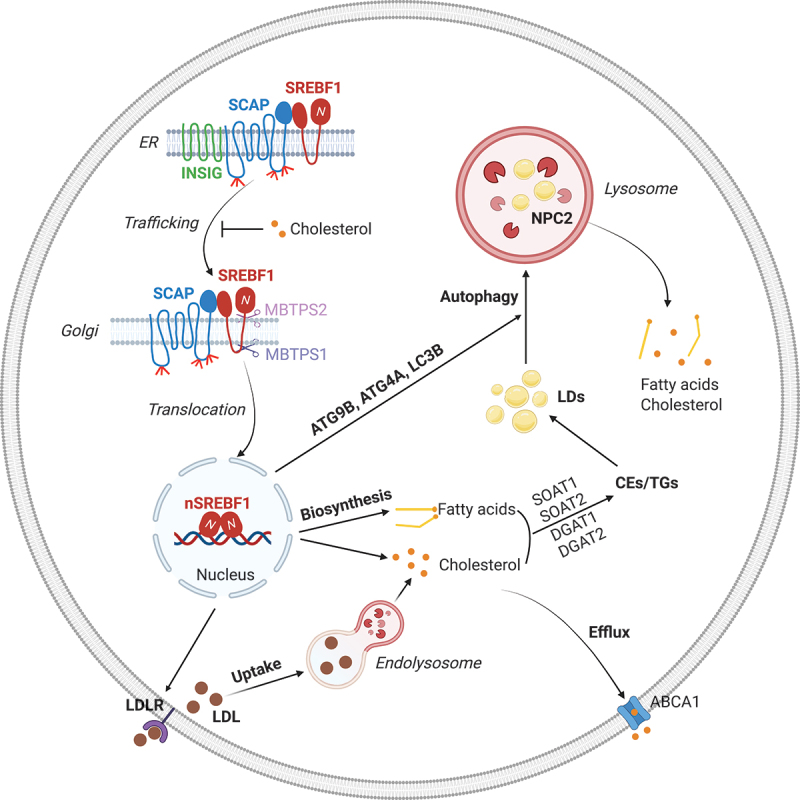
SREBF1 concurrently regulates lipid synthesis and lipophagy to maintain lipid homeostasis needed to promote tumor growth. After synthesis, SREBF1 is restrained in the endoplasmic reticulum (ER) by INSIG (insulin induced gene), which binds to SCAP (SREBF chaperone) to form the INSIG-SCAP-SREBF1 complex. Upon cholesterol reduction, SCAP dissociates from INSIG and transports SREBF1 from the ER to the Golgi, where SREBF1 is sequentially cleaved by MBTPS1/S1P and MBTPS2/S2P. This cleavage allows for the release of the SREBF1 N-terminal active form that then enters the nucleus to promote a diverse array of gene expression, including LDLR (low density lipoprotein receptor), cholesterol and fatty acid synthesis-related genes and autophagy-related genes ATG9B, ATG4A and LC3B, as well as NPC2 (NPC intracellular cholesterol transporter 2). LDLR mediates LDL entry into cells, which is then hydrolyzed in the lysosomes to release carried cholesterol and fatty acids to support tumor cell growth. Excess cholesterol and fatty acids are converted to cholesterol esters (CEs) and triglycerides (TGs), respectively, and then stored in lipid droplets (LDs). Excess cholesterol can also be exported outside of cells by ABCA1 (ATP binding cassette subfamily a member 1). Under conditions of nutrient deprivation, LDs are mobilized into lysosomes by an autophagic process (i.e., lipophagy) that releases the stored cholesterol and fatty acids to promote tumor cell growth. Thus, LDs serve as a critical reservoir to maintain proper cellular lipid levels, and they prevent excess free cholesterol and fatty acids from triggering cellular toxicity. The figure was created with BioRender.com.
