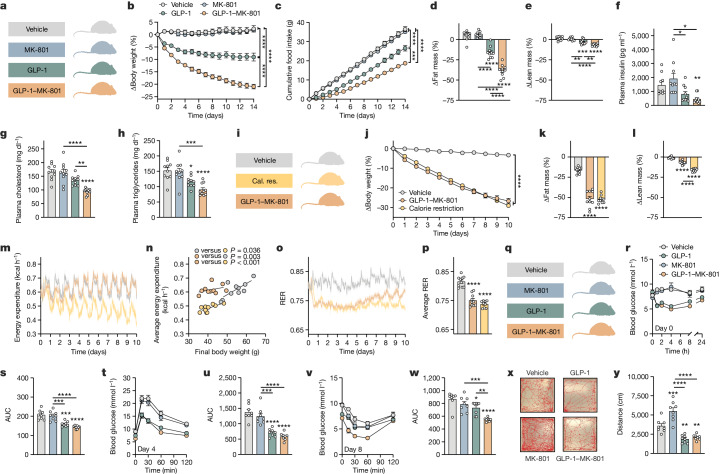
Fig. 1. GLP-1–MK-801 corrects metabolic disease.
a–h, DIO mice were treated once-daily with s.c. injections of MK-801, GLP-1, GLP-1–MK-801 or vehicle for 14 days. n = 10 mice per group. 100 nmol kg−1 dose. a, Schematic. b, Change in body weight. c, Cumulative food intake. d, Change in fat mass. e, Change in lean mass. f, Plasma insulin. g, Plasma cholesterol. h, Plasma triglycerides. i–p, DIO mice were treated once-daily with s.c. injections of GLP-1–MK-801 (100 nmol kg−1), calorie restriction (cal. res.) to match the weight loss of the GLP-1–MK-801 group or vehicle for 10 days. n = 9–10 mice per group. i, Schematic. j, Change in body weight. k, Change in fat mass. l, Change in lean mass. m, Energy expenditure. n, Average energy expenditure relative to final body weight. o, RER. One mouse in the calorie-restriction group was excluded due to a CO2-sensor-related deviation. p, Average RER. q–w, DIO mice were treated once-daily with s.c. injections of MK-801, GLP-1, GLP-1–MK-801 or vehicle for 8 days. n = 8 mice per group. 100 nmol kg−1 dose. q, Schematic. r, Compound tolerance test on day 0. s, Area under the curve (AUC) of data in r. t, Glucose tolerance test on day 4. u, AUC of data in t. v, Insulin tolerance test on day 8. w, AUC of data in v. x,y, Open-field test after a single s.c. injection of MK-801, GLP-1, GLP-1–MK-801 or vehicle. n = 8 mice per group. 300 nmol kg−1 dose. x, Representative traces. y, Distance travelled. Data were analysed using one-way analysis of variance (ANOVA) with Bonferroni post hoc multiple-comparison test (d–h, k, l, p, s, u, w and y), two-way repeated-measures ANOVA to assess main effects of treatment (b, c and j) or analysis of covariance (ANCOVA) computed with calR using body weight as a covariate (n). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Detailed statistics are provided in Supplementary Table 1. The diagrams in a, i and q were created using BioRender.