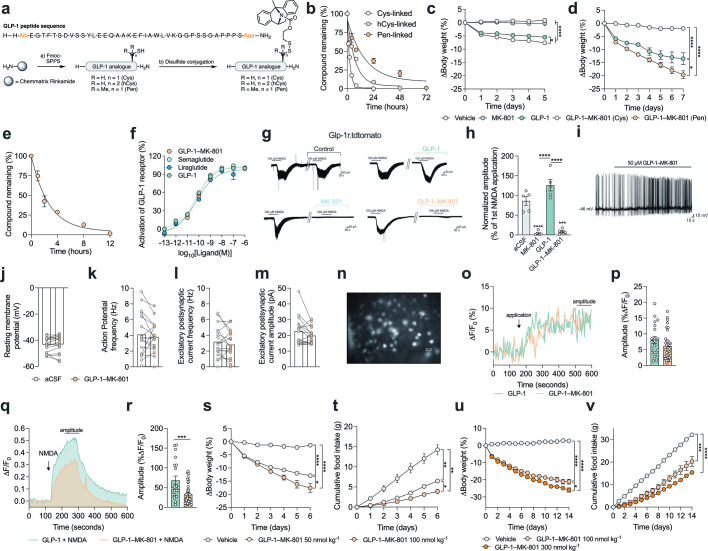
Extended Data Fig. 2. Pharmacological characterization of GLP-1–MK-801.
a, Chemical synthesis of GLP-1–MK-801 conjugates with different cysteine homologues. b, In vitro stability assay in human plasma. The assay was performed as biological replicates of GLP-1–MK-801 (Cys-linked) (n = 3), GLP-1–MK-801 (hCys-linked) (n = 2), GLP-1–MK-801 (Pen-linked) (n = 3). c, Change in body weight of DIO mice treated with once-daily s.c. injections of 100 nmol kg−1 MK-801 (n = 8 mice), 100 nmol kg−1 GLP-1 (n = 8 mice), 100 nmol kg−1 GLP-1–MK-801 (Cys-linked) (n = 8 mice) or vehicle (isotonic saline, n = 8 mice) for 5 days. d, Change in body weight of DIO mice treated with once-daily s.c. injections of 100 nmol kg−1 GLP-1 (n = 6 mice), 100 nmol kg−1 GLP-1–MK-801 (Pen-linked) (n = 6 mice) or vehicle (isotonic saline, n = 6 mice) for 7 days. e, Degradation assay. GLP-1–MK-801 was incubated in PBS with 200 mM glutathione at pH 7.0. The assay was performed as triplicates (n = 3). f, Murine GLP-1 receptor activation of GLP-1, GLP-1–MK-801, liraglutide and semaglutide in transiently transfected HEK293 cells. The data represents dose-response at 2 min and is normalized to the maximal GLP-1 response (100%). The assay was performed as duplicates (n = 2). g, h, Electrophysiological recordings of GLP-1 receptor-positive neurons, which were identified using a tdtomato reporter, being stimulated with NMDA after 30 min of bath application of GLP-1 or GLP-1–MK-801. g, Current responses at a holding potential of −70 mV elicited by NMDA bath application in GLP-1 receptor-positive neurons of the arcuate nucleus (ARC) with bath application of 50 µM GLP-1–MK-801, 50 µM GLP-1, 50 µM MK-801 or artificial cerebrospinal fluid (aCSF). h, Bar graph summarizing the effect of NMDA-induced inward current after control (aCSF, n = 5 neurons), GLP-1 (50 μM, n = 5 neurons), MK-801 (50 μM, n = 5 neurons) or GLP-1–MK-801 (50 μM, n = 5 neurons). Bath application normalized to the first bath application of NMDA (100 μM). i-m, Electrophysiological recordings of POMC neurons stimulated with GLP-1–MK-801. i, Representative trace showing that 50 μM GLP-1–MK-801 acute bath application induces a depolarization of POMC neurons in ARC (n = 6 of 17 neurons). Bar graphs summarizing the acute effect of 50 μM GLP-1–MK-801 on POMC neurons. j, Resting membrane potential, k, Action potential frequency n. l, Excitatory postsynaptic current (EPSC) frequency. m, EPSC amplitude of POMC neurons that were depolarized in response to GLP-1–MK-801 bath application. n-r, Calcium imaging of ARC brain slices. n, Representative image of Fura-2AM loaded cells in an ARC brain slice. Scalebar is 20 µm. o, Application of GLP-1 and GLP-1–MK-801 induced changes in fluorescence indicative of rises in intracellular calcium (%ΔF/F0) as shown by these representative fluorescent responses of two different Fura 2-AM loaded cells to application of GLP-1 (1 µM) or GLP-1–MK-801 (1 µM). p, The amplitude of the change in fluorescence (%ΔF/F0) elicited by GLP-1 (1 µM, n = 20 neurons) or GLP-1–MK-801 (1 µM, n = 38 neurons). q, Representative traces of intracellular calcium levels in response to application of NMDA (50 µM) following bath application of GLP-1 (1 µM) or GLP-1–MK-801 (1 µM). r, Quantification of NMDA-induced (50 µM) intracellular calcium rises (%ΔF/F0) following bath application of GLP-1 (1 µM, n = 20 neurons) or GLP-1–MK-801 (1 µM, n = 38 neurons). s, t, Treatment of DIO mice with once-daily s.c. injections of 50 nmol kg−1 GLP-1–MK-801 (n = 6 mice and n = 3 cages), 100 nmol kg−1 GLP-1–MK-801 (n = 6 mice and n = 3 cages) or vehicle (isotonic saline, n = 6 mice and n = 3 cages) for 6 days. s, Change in body weight. t, Cumulative food intake. u, v, Treatment of C57BL/6 J DIO mice with once-daily s.c. injections of 100 nmol kg−1 GLP-1–MK-801 (n = 8 mice and n = 4 cages), 300 nmol kg−1 GLP-1–MK-801 (n = 8 mice and n = 4 cages) or vehicle (isotonic saline, n = 8 mice and n = 4 cages) for 14 days. u, Change in body weight. v, Cumulative food intake. Data analysed by paired two-tailed Student’s t-test (j-m), unpaired two-tailed Student’s t-test (p and r), one-way ANOVA, multiple comparison, Bonferroni post hoc test (h) and two-way repeated measures ANOVA to assess main effects of treatment (c, d and s-v). Data represents mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Detailed statistics are in Supplementary Table 1.