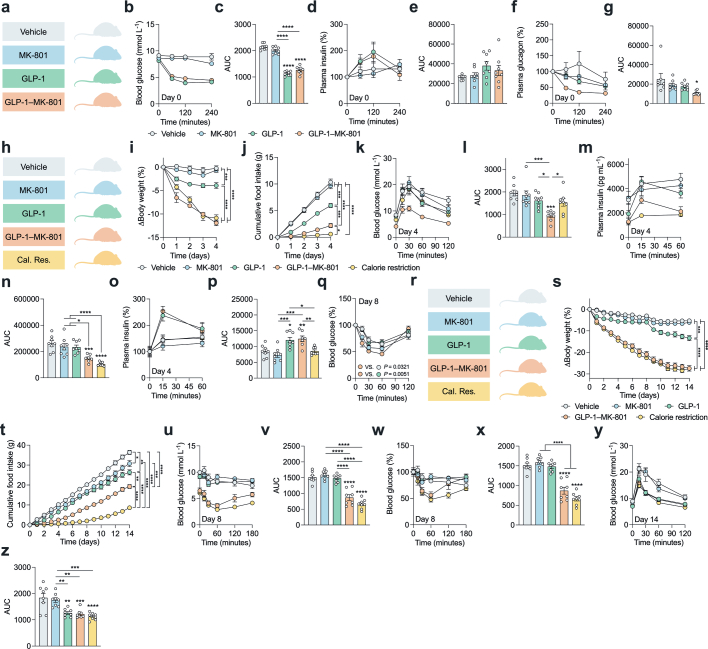
Extended Data Fig. 4. Evaluation of glucometabolic effects of GLP-1–MK-801.
a-g, Compound tolerance test in response to a single s.c. injection of 100 nmol kg−1 MK-801 (n = 8 mice), 100 nmol kg−1 GLP-1 (n = 8 mice), 100 nmol kg−1, GLP-1–MK-801 (n = 8 mice), or vehicle (isotonic saline, n = 8 mice) in DIO mice. a, Schematic. b, Compound tolerance test on day 0. c, AUC of b. d, Plasma insulin levels during compound tolerance test. One mouse and one datapoint in vehicle group was omitted from analysis as they had insulin levels outside the assay range. e, AUC of d. f, Plasma glucagon levels during compound tolerance test. One mouse in vehicle group had supraphysiological glucagon levels outside the assay range and was omitted from analysis. g, AUC of f. h-p, Glucose-stimulated insulin secretion. Treatment of DIO mice with once-daily s.c. injections of 100 nmol kg−1 MK-801 (n = 8 mice and n = 4 cages), 100 nmol kg−1 GLP-1 (n = 8 mice and n = 4 cages), 100 nmol kg−1 GLP-1–MK-801 (n = 8 mice and n = 4 cages), calorie restriction (Cal. Res.) to match the weight loss of GLP-1–MK-801 (n = 8 mice and n = 5 cages) or vehicle (isotonic saline, n = 8 mice and n = 5 cages) for 4 days. On day 4, an intraperitoneal glucose tolerance test was conducted with collection of tail blood at timepoints 0, 15 and 60 min. h, Schematic. i, Change in body weight. j, Cumulative food intake. k, Glucose tolerance test on day 4. l, AUC of k. m, Glucose-stimulated insulin secretion. One datapoint in vehicle group, one datapoint in MK-801 group and one mouse and one datapoint in GLP-1–MK-801 group were outside assay range and omitted from analysis. n, AUC of m. o, Percentage to baseline of m. p, AUC of o. q, Percentage to baseline of insulin tolerance test in Fig. 1v. r-z, Insulin and glucose tolerance compared to mice undergoing calorie restriction to match the weight loss trajectory of GLP-1–MK-801. Treatment of DIO mice with once daily s.c. injections of 100 nmol kg−1 MK-801 (n = 8 mice and n = 4 cages), 100 nmol kg−1 GLP-1 (n = 8 mice and n = 4 cages), 100 nmol kg−1 GLP-1–MK-801 (n = 8 mice and n = 4 cages), calorie restriction (Cal. Res.) to match the weight loss of GLP-1–MK-801 (n = 8 mice and n = 4 cages) or vehicle (isotonic saline, n = 8 mice and n = 3-4 cages) for 14 days. On day 8, an intraperitoneal insulin tolerance test was conducted and on day 14 an intraperitoneal glucose tolerance test was conducted. r, Schematic. s, Change in body weight. t, Cumulative food intake. Four datapoints were excluded for one cage in vehicle group due to food spillage on day 11-14. u, Insulin tolerance test on day 8. v, AUC of u. w, Percentage to baseline of u. x, AUC of w. y, Glucose tolerance test on day 14. z, AUC of y. Data analysed by one-way ANOVA, multiple comparison, Bonferroni post hoc test (c, e, g, l, n, p, v, x and z) and two-way repeated measures ANOVA to assess main effects of treatment (i, j, q, s and t). Data represents mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Detailed statistics are in Supplementary Table 1. The diagrams in a, h and r were created using BioRender.