Abstract
Multiple sclerosis (MS) is characterized by progressive neuroinflammation and neurodegeneration from disease onset that, if left untreated, can result in the accumulation of irreversible neurological disability. Early intervention with high-efficacy therapies (HETs) is increasingly recognized as the best strategy to delay or mitigate disease progression from the earliest stages of the disease and to prevent long-term neurodegeneration. Although there is growing clinical and real-world evidence supporting early HET intervention, foregoing this strategy in favor of a traditional escalation approach prioritizing lower-efficacy disease-modifying therapies remains a common approach in clinical practice. This review explores potential health care professional- and patient-related barriers to the early use of HETs in patients with MS in the United States. Barriers can include regulatory and reimbursement restrictions; knowledge gaps and long-term safety concerns among health care professionals; and various individual, cultural, and societal factors affecting patients. Potential strategies for overcoming these barriers and encouraging early HET use are proposed.
Keywords: Multiple sclerosis, High-efficacy therapies, Disease-modifying therapies, United States, Shared decision-making, Health care professionals
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated neuroinflammatory and neurodegenerative disease of the central nervous system (CNS) [1]. MS is traditionally categorized into clinically isolated syndrome, relapsing–remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS) [1], but there is increasing evidence that these subtypes form a continuous spectrum, with blurred boundaries between relapsing and progressive subtypes [2, 3]. MS disease activity and underlying neuroinflammation activity are typically indicated through clinical relapses, magnetic resonance imaging (MRI) activity, and worsening disability [4].
Because inflammatory disease activity and irreversible neurodegeneration can occur early in the disease course, even before the first clinical event [5, 6], early therapeutic intervention is recognized as giving the best long-term prognosis, compared with delayed or no intervention [7–9]. Despite this, many people with MS do not receive appropriate timely treatment, which can lead to poor disability outcomes [3, 10]. Selecting the optimal disease-modifying therapy (DMT) for newly diagnosed patients with MS is complex because our ability to precisely predict the MS disease course is limited. This is further complicated by drug-specific factors, such as mechanism of action, route of administration, dosing schedule, efficacy, and safety profile [11, 12]. Real-world evidence is emerging that early intervention with high-efficacy therapies (HETs) may provide the best opportunity to protect the CNS against irreversible injury and substantially mitigate the early inflammatory component of disease [13–17]. Until ongoing randomized controlled trials are able to confirm this stance from the clinical perspective [18, 19], there remains no formal consensus on the best therapeutic approach to MS care. Accordingly, those who may benefit from receiving HETs early in the disease course are likely to face more barriers to HET access than with traditional DMTs. These include restrictions imposed by reimbursement bodies; access to MS specialist neurologists and clinics; and patient-specific considerations, including socioeconomic background, ethnicity, geographic location, health insurance status, physical disability, comorbidities, long-term safety concerns, and family planning [7].
This review explores the obstacles to an early HET intervention strategy as related to health care professionals (HCPs) and patients with MS in the United States (US). The rationale and challenges of early HET adoption from the European perspective have been discussed elsewhere [10]. We also discuss potential strategies for overcoming these challenges and improving care for people with MS in the US who are underserved.
Classification of DMTs by efficacy
For the purposes of this review and in lieu of comprehensive comparative trials, we have classified DMTs by efficacy into three categories based on their ability to reduce annualized relapse rates (ARRs) in phase 3 clinical trials. ARR was the primary outcome in a majority of these trials and has been used in similar DMT categorization approaches [20–23]. Low-efficacy therapies reduce ARR by 20–40% vs placebo in clinical trials and comprise the first-generation injectables interferon (IFN) beta and glatiramer acetate, and the second-generation oral medication teriflunomide (Table 1). Moderate-efficacy therapies reduce ARR by 40–60% vs placebo or by < 50% vs active comparator in clinical trials and include fumarates, cladribine, and the sphingosine-1-phosphate (S1P) receptor modulators (fingolimod, siponimod, ozanimod, and ponesimod). We have defined HETs as those that have reduced ARR by ~ 50% or more vs active comparator or by > 65% vs placebo in clinical trials; these include the US Food and Drug Administration–approved monoclonal antibodies for use in MS: natalizumab, alemtuzumab, ocrelizumab, ofatumumab, and ublituximab [8, 22, 24–26]. Other meta-analyses have similarly ranked monoclonal antibody DMTs, oral therapies (except teriflunomide), and then glatiramer acetate, interferons, and teriflunomide in descending order of efficacy based on their ARR vs placebo [22, 23].
Table 1.
Efficacy of DMTs for MS according to reduction in ARR relative to active comparator or placebo, as determined from the approved dose in pivotal clinical trials
| DMT, class | Comparator | Clinical trial | Reduction of ARR | |
|---|---|---|---|---|
| High efficacya | Anti-CD20 monoclonal antibody | |||
| Ocrelizumab [27] | IFN beta-1a SC 44 mg | OPERA I/II | 46–47% | |
| Ofatumumab [28] | Teriflunomide 14 mg | ASCLEPIOS I/II | 50–60% | |
| Ublituximab [29] | Teriflunomide 14 mg | ULTIMATE I/II | 50–58% | |
| Anti-CD52 monoclonal antibody | ||||
| Alemtuzumab [30, 31] | IFN beta-1a SC 44 mg | CARE-MS I/II | 49–55% | |
| Anti-a4 integrin receptor monoclonal antibody | ||||
| Natalizumab [32] | Placebo | AFFIRM | 68% | |
| Moderate efficacyb | S1P receptor modulators | |||
| Fingolimod [33, 34] | Placebo | FREEDOMS I/II | 48–54% | |
| Siponimod [35] | Placebo | EXPAND | 55% | |
| Ozanimod [36, 37] | IFN beta-1a IM 30 mg |
RADIANCE/ SUNBEAM |
21–48% | |
| Ponesimod [38] | Teriflunomide 14 mg | OPTIMUM | 31% | |
| Purine analog | ||||
| Cladribine [39] | Placebo | CLARITY | 58% | |
| Fumarates | ||||
| Dimethyl fumarate [40, 41] | Placebo | CONFIRM/DEFINE | 44–53% | |
| Diroximel fumarate [42] | EVOLVE-MS-1 | |||
| Low efficacyc | Pyrimidine synthesis inhibitor | |||
| Teriflunomide [43, 44] | Placebo | TOWER/TEMSO | 31.5–36% | |
| Amino acid copolymer | ||||
| Glatiramer acetate [40, 45] | Placebo | CONFIRM/GALA | 29–34% | |
| IFNs | ||||
| IFN beta-1a [46] | Placebo | PRISMS | 33% | |
ARR annualized relapse rate, DMT disease-modifying therapy, HET high-efficacy therapy, IFN interferon, IM intramuscular, MS multiple sclerosis, S1P sphingosine-1-phosphate, SC subcutaneous
aHETs reduce the ARR by ~ 50% or more vs active comparator or > 65% vs placebo
bModerate-efficacy therapies reduce the ARR by 40–60% vs placebo or < 50% vs active comparator
cLow-efficacy therapies reduce the ARR by 20–40% vs placebo
An important note is that this classification based on ARR does not account for DMT effects on MRI, disability, or other disease measures, and certain DMTs might be more effective on measures other than ARR. For instance, S1P modulators and cladribine are considered moderate-efficacy DMTs according to the above definition and non-HETs in other publications yet have also been defined as HETs elsewhere [9, 13–15, 47]. Categorizing higher-efficacy DMTs using data from active comparator studies can therefore enable more granulated delineation between high and moderate efficacy.
Existing treatment paradigms
An escalation strategy is one in which patients initially receive lower-efficacy therapies with well-characterized safety profiles to minimize concerns about side effects [48, 49]. HCPs then switch patients to higher-efficacy therapies following breakthrough disease activity (i.e., new clinical relapses and/or MRI activity) [48, 49].
Initial intervention with lower-efficacy therapies might benefit some patients, such as those with a mild disease course or a preference for lower-risk DMTs [50]. Escalation might also allow some patients flexibility with treatment options, taking into account prognostic measures, risk tolerance, age, and available financial resources [24]. Because lower-efficacy DMTs are thought to be more immunomodulatory than immunosuppressive [51], they can be particularly useful for patients with comorbidities, such as chronic infections, where immunosurveillance is essential. The clinical data suggest that low-efficacy therapies may have value in reducing inflammation, mild relapses, and disability progression in the beginning of the disease; however, their effect on accumulation of disability may not be maintained long-term [52–54].
Society guidelines have traditionally recommended an escalation approach [55, 56]. Patient care is now evolving, with many MS specialists and other clinicians prioritizing treatment goals of mitigating or halting the underlying inflammatory mechanisms of MS to prevent irreversible disability [8, 47]. This has led to a shift toward an early aggressive or proactive treatment approach, where patients are initiated on HETs (often at diagnosis) to more effectively prevent relapses, reduce potential neuronal injury, slow disability accrual, and ultimately improve optimal patient outcomes [8, 48].
Efficacy and safety of lower-efficacy therapies
Clinical trials of patients with RRMS have revealed comparable reductions in ARR for IFNs, glatiramer acetate, and teriflunomide and largely similar effects on time to first relapse for IFNs and glatiramer acetate (Table 1) [40, 43–46, 57, 58]. Both teriflunomide and IFN therapy have been reported to delay disability progression [43, 46, 59, 60], whereas glatiramer acetate appears to have no significant impact other than a potential stabilizing effect of long-term disability progression in patients with mild disease activity [45, 53].
In terms of their safety profiles, IFNs and glatiramer acetate are generally well tolerated and are most commonly associated with injection-site reactions [11]. Both are considered safe for use during pregnancy, have a perceived lower risk of infections compared with higher-efficacy therapies, and only minimally affect immune responses to vaccines [26, 61]. Common adverse events (AEs) with teriflunomide include hypertension, diarrhea, and hair loss [59, 62]. Regular liver function testing and use of effective contraception is advised for teriflunomide as it carries a black box warning for severe liver injury and teratogenicity, although recent post-marketing pregnancy registry data suggest variable outcomes [62–65]. Teriflunomide is eliminated slowly from the plasma, and a wash-out with cholestyramine prior to switching to another DMT is advisable because it can remain in the blood for up to 2 years after the last dose [62].
In patients with RRMS, S1P modulators, dimethyl fumarate, and cladribine led to greater reductions in ARR vs low-efficacy therapies or placebo (Table 1) [36–41, 66, 67]. Although these moderate-efficacy therapies are mostly well tolerated, some notable safety considerations include warnings of malignancy and teratogenicity with cladribine [68]; gastrointestinal AEs with dimethyl fumarate [40]; and an increased risk of cardiovascular events, macular edema, and rare serious opportunistic infections, such as progressive multifocal leukoencephalopathy (PML) and cryptococcal meningitis with S1P receptor modulators [69–72].
The need for effective therapies in early MS
Disability in MS is one of the main drivers of poor quality of life among people with MS [73–76]. Disability accrual occurs primarily via two mechanisms: relapse-associated worsening (RAW) due to acute lesions and incomplete recovery from relapses and progression independent of relapse activity (PIRA) encompassing the gradual clinical progression from disease onset [77]. RAW is a form of acute neuroinflammation that appears to be driven by peripherally activated B cells and T cells, whereas PIRA (also known as “smoldering inflammation”) can be primarily attributed to pathogenic microglia in the CNS that drive progressive neuroaxonal loss [78, 79]. Meningeal lymphoid aggregates and subpial cortical lesions contribute to neurodegeneration [80]. Chronically active MS lesions (including paramagnetic rim lesions), characterized by macrophage-mediated injury, contribute to smoldering inflammation and potentially PIRA pathogenesis [78, 80]. PIRA plays a significant role in disease worsening and, over time, may become the main contributor to disability progression [77, 81]. Because current DMTs are limited in their abilities to stop PIRA, early and effective intervention may therefore be the optimal strategy to prevent or delay long-term irreversible disability.
Two ongoing prospective, randomized pragmatic trials (TREAT-MS [NCT03500328] and DELIVER-MS [NCT03535298] [18, 19]) are directly comparing early HET vs escalation therapy in terms of disability progression, relapses, neurodegeneration, health-related quality of life, burden of MS, cognition, employment, and safety. Data from these trials will shed more light on the wider benefit of early HET to the patient, beyond clinical efficacy and safety measures.
Benefits and safety considerations of early HET
Efficacy and risk of disability progression
The question of whether early HET or escalation therapy delivers the best long-term outcomes for patients with MS hinges on conclusive evidence from randomized controlled trials for there to be any consensus among MS neurologists. While DELIVER-MS and TREAT-MS aim to definitively answer this question, some extrapolations can be made from pivotal comparator-controlled trials of HETs in treatment-naïve patients. In the CARE-MS I trial of patients with early RRMS, alemtuzumab led to reduced relapse rates, slower brain volume loss, and more patients remaining free from clinical disease activity compared with IFN beta-1a [30]. Similarly, natalizumab substantially reduced the risk of disability progression and relapse rate in patients with highly active RMS in the AFFIRM and SENTINEL trials [82]. The OPERA I/II and ORATORIO clinical trials of ocrelizumab enrolled patients with RMS and PPMS, respectively, most of whom had not received DMTs in the previous 2 years. Both trials demonstrated improved efficacy outcomes (ARR, disability progression, lesion burden, physical and cognitive scores) vs IFN beta or placebo [27, 83]. In recently diagnosed patients with relapsing MS from the ASCLEPIOS I/II clinical trials, ofatumumab led to significantly reduced ARR, fewer MRI lesions, and increased odds of achieving “no evidence of disease activity” vs teriflunomide [84]. In the ULTIMATE I and II clinical trials, patients with RMS treated with ublituximab experienced a lower ARR and fewer brain lesions on MRI than with teriflunomide but no significantly lower risk of disability progression [29]. The effect of HET on relapse rates in clinical trials implies protection against RAW, but what about PIRA? In ASCLEPIOS I/II, ofatumumab significantly reduced the risk of experiencing PIRA events over a 6-month period compared with teriflunomide [84]. Likewise, OPERA I/II found ocrelizumab to be superior to IFN beta-1a in preventing PIRA over 12 and 24 weeks [81]. However, the question of whether mitigating early PIRA events can prevent long-term neurodegeneration is beyond the scope of these trials.
Real-world evidence has revealed clinical benefits, lower disease progression, and favorable long-term outcomes for patients receiving early HET vs escalation therapy or delayed HET [13, 15, 85–87]. In an observational study using data from the MSBase and Swedish MS registries, HET treatment initiated within 2 years of disease onset reduced the risk of disability progression by 66% after 6–10 years compared with HET initiated later in the disease course (hazard ratio 0.34; 95% confidence interval, 0.23, 0.51) [14]. Two similar studies comparing data from Danish, Czech, and Swedish MS registries found that the Swedish high-efficacy induction strategy resulted in reduced risk of disability progression and relapses compared with the Danish and Czech escalation strategies [16, 88]. Further, a systematic review of seven studies revealed that early HET had a 30% reduction in disability worsening at 5 years vs escalation therapy [89]. It should be noted that because some real-world studies include S1P modulators in their definition of high efficacy [16, 85, 86]; the true beneficial effects of HETs as characterized in this review may be underestimated depending on the respective HET definitions of each study.
Patients who received HET on the basis of more active disease actually had a lower long-term risk of conversion to SPMS than those with less active disease on escalation therapy [85]. Even after patients switched from moderate-efficacy therapy to HET, those who initiated early HET showed improved longer-term outcomes compared with those on delayed HET [14, 86]. Younger age is a major factor of immunomodulatory drug efficacy [90], likely due to higher cerebral reserve, further reinforcing the importance of early treatment. Overall, the available data indicate that patients can achieve maximum therapeutic benefit with early HET therapy, regardless of prognostic factors or disease severity [10, 91].
Safety and risk of AEs
Patients receiving HETs do not necessarily experience more AEs than those receiving lower-efficacy therapies. In an observational study of 4861 patients, the proportion of patients discontinuing treatment due to AEs was comparable between those on an early HET strategy (where one-third of patients received primarily first-line rituximab or natalizumab) and those on an escalation strategy (where nearly all patients received first-line low- or moderate-efficacy therapy, mostly teriflunomide) [16]. Similarly, a systematic review of two studies reported a similar safety profile between early HET and escalation strategies, with comparable proportions of serious AEs [89]. The OPERA, ASCLEPIOS, and ULTIMATE trials further showed that, aside from infusion- or injection-related reactions, the safety profiles of ocrelizumab, ofatumumab, and ublituximab were generally similar to the lower-efficacy therapies IFN beta and teriflunomide [27, 29, 84]. Low serum immunoglobulin (Ig) levels can occur with lymphocyte-depleting HETs and have been associated with increased infection risk [92–94]. However, in patients treated with ofatumumab for up to 5 years, mean immunoglobulin (Ig)G levels remained stable and mean IgM levels decreased but remained above the lower limit of normal [95]. Although no link between reduced Ig levels and risk of serious infection was found [95], this potential risk can nonetheless be mitigated by careful laboratory and clinical monitoring [26].
Longer-term safety analyses of HETs have been reported, although this is currently limited for newer HETs, such as ublituximab. Infusion-related reactions, opportunistic infections, and serious infections remain among the most frequently reported AEs [96–99]. Natalizumab treatment has been associated with increased incidence of PML (estimated to occur at rates of 0.01–10 per 1000 individuals with John Cunningham virus positivity) with increasing risk depending on the duration of treatment (especially over 2 years) and prior immunosuppressant therapy [99–101]. Safety data for ofatumumab for ≤ 3.5 years or ocrelizumab for ≤ 5 years have not shown increases in the incidence and risks of AEs over those reported in the clinical trials [97, 98]. Alemtuzumab is associated with secondary autoimmune disease (particularly thyroid disorders) that can occur post treatment with delayed onset, although the risk decreases in the fourth year after the last dose [96, 102]. Serious safety considerations for alemtuzumab include the risk of infusion-related ischemic and hemorrhagic stroke and cervicocephalic arterial dissection [103]. Longer-term safety analyses indicate that the incidence of AEs, such as infusion-related reactions and infections, reduce over time with ongoing HET treatment [96, 97, 99, 102]. Careful monitoring, patient education, and risk mitigation can facilitate early detection and effective management of AEs [96, 104]. Taken together, the emerging long-term data on HETs suggest that these therapies should not be excluded solely on the basis of their safety profiles, but consideration of individual patient factors is warranted. There remains an unmet need for robust long-term data comparing the safety of higher- and lower-efficacy therapies to fully address perceived safety risks with HET treatment.
Socioeconomic benefits of early treatment
MS-related disability progression can have deleterious effects on society and the economy. Disability is a chief driver of costs, which greatly increase as disability level increases [76, 105]. Dependency on medication and health care resources, in addition to increasing usage of informal care, substantially contribute to these rising expenses [105]. The indirect costs of care and loss of productivity of patients and their caregivers are responsible for the greatest financial burden in MS [106]. The largest contributors to these costs are lost earnings due to presenteeism (defined as presence at work without productivity), absenteeism, and premature death [107]. The MS Cost of Illness Study found that the probability of working, work hours, and work productivity all reduced with increasing subjective cognitive impairment and fatigue [108]. In particular, MS-related fatigue is a highly prevalent symptom in clinical practice and affects approximately 80% of people with MS [109–111], although figures of > 90% have been reported [111]. In people with MS, presenteeism and absenteeism have been linked to physical and cognitive fatigue, symptom severity, depression, anxiety, and disability [112, 113].
Cost-effective analyses of early vs delayed initiation of HET in MS confirm a positive socioeconomic impact of early intervention. The lower overall costs from reducing disability with early HET can compensate for the initial expense of the medications [114, 115]. Lower incidence of relapses and delaying or preventing disease progression could lead to a decrease in health care resource utilization and associated costs [116–118]. In fact, early HET can be more cost-effective than an escalation approach at reducing disability progression within a 5-year period [89]. As a result of improving patient health, effective therapeutic intervention from diagnosis could mitigate the societal and economic burden of disease.
Barriers to adoption of early HETs and potential solutions
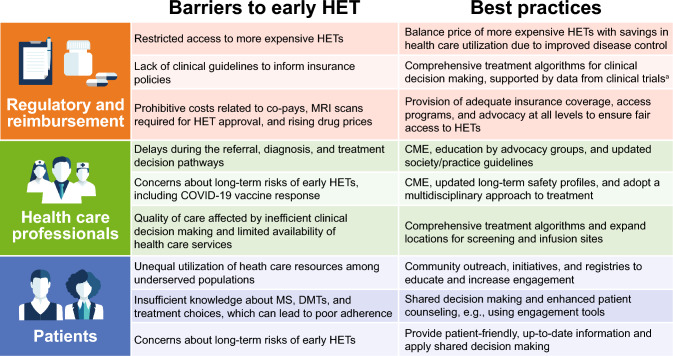
Analyses of US treatment patterns including data up to 2020 have revealed a rising number of HET initiations over time [119, 120], an upward trend that market growth forecasts indicate is likely to continue. Despite this, moderate-efficacy oral therapies were initiated most often up to 2020, by a substantial margin [120]. More up-to-date reports on current HET usage are limited in part due to the delayed availability of real-world data capturing more recently approved HETs (i.e., ofatumumab, ublituximab). Nonetheless, while it can be assumed that even more patients in the US will receive HET treatment in the coming years, difficulties in accessing this treatment are likely to remain for the individual. Social and cultural factors affecting access to care, insurance company requirements, financial burdens, and perceptions of risk are just a few well-documented examples [121–123]. The issue is further compounded by a lack of clear guidelines on initiating HET early in the disease course that could preclude many first-line treatment options. The potential challenges and proposed solutions to strengthen the early HET approach are outlined in the following sections and summarized in Fig. 1.
Fig. 1.
Proposed best practices for overcoming barriers to early high-efficacy therapy (HET) treatment in multiple sclerosis (MS) from the perspective of regulatory and reimbursement agencies, health care professionals, and patients. aAnticipated clinical trial data from DELIVER-MS and TREAT-MS to support the benefit of early HET treatment in MS outcomes. CME continuing medical education, COVID-19 coronavirus disease 2019, DMT disease-modifying therapy, MRI magnetic resonance imaging
Regulatory and reimbursement factors
Conflicting priorities between insurance companies and patients is a significant contributor to disparities in access to MS treatments. US insurance companies can restrict or deny access to some DMTs (despite their regulatory approval) and often insist that lower-efficacy therapies be used first [124, 125]. High rates of medication denials occur in both commercial and government (i.e., Medicaid) insurance programs and increase the likelihood of disease activity [125]. Because the initial higher costs of HETs impact the short-term budgets of payers, the long-term indirect and societal benefits (e.g., improved productivity) of curbing disability accrual early may be considered beyond the remit of payers’ budgets [10]. Long-term cost-savings could therefore be lost in favor of short-term budget needs. Policy development from governmental and private insurers could ease these restrictions but is hindered by a lack of consensus from the MS community regarding optimal treatment and concerns about rising DMT costs [49, 126]. Results from the ongoing DELIVER-MS and TREAT-MS trials [18, 19] should be able to inform the development of national consensus statements and treatment algorithms necessary for policy reform. Concurrently, effective communication must be established with legislative decision makers and insurers to discuss DMT safety profiles and the clinical, financial, and societal consequences of poor disease control [10].
In addition to changes in insurance coverage, patient access to treatment in the US is impacted by unaffordable co-pays and out-of-pocket expenses from Medicare and commercial benefit designs [126–128]. A NARCOMS Registry survey exploring the effects of health insurance on DMT usage in MS revealed that 6.1% of patients with MS and specifically 9.2% with RRMS were unable to take DMTs due to insurance or financial reasons [126]. Overall, 7.8% of patients who obtained DMTs through insurance faced at least one insurance challenge, such as initial denial of their DMT use [126]. Personal finance considerations and challenges with co-pays influenced DMT usage, with approximately 25% of patients partially or completely relying on support from free or discounted drug programs [126]. The entry of more affordable generic and biosimilar medications into the market (such as those for dimethyl fumarate, fingolimod, teriflunomide, and natalizumab) attempts to counterbalance the expensive prescription drug market, but HET access is still restricted due to the stepwise approach of many insurance policies [129]. For patients covered by Medicare, generics do not necessarily offer budgetary relief as generic manufacturers are not required to provide discounts in the coverage gap, which normally contribute to a person’s total out-of-pocket spending [130].
There is clear need to alleviate the burden on patients trying to access appropriate MS care. Insurance company policies on DMT coverage that allow patients early access to the right treatment for them would provide substantial relief [124]. Pharmaceutical companies could be proactive in identifying patients with financial barriers and enroll them into patient assistance programs to help with drug costs and co-pays. Collaboration among neurologists, MS organizations, and patient advocacy groups may help to ensure fair access to DMTs; indeed, patients are already campaigning to address these issues [124, 131]. Involving regulatory authorities and health technology assessors could also help to facilitate cooperation from insurance companies [7].
In addition to prohibitive therapy costs, access to timely MRIs during the diagnostic and surveillance stages is further limited by out-of-pocket costs for the patient; the national average cost for an MRI in the US is $1325, which rises to $2250 in a hospital setting [132]. Moreover, variability and poor standardization in MRI protocols, machine and image quality, radiologist expertise, and personnel training in clinical practice can result in an inaccurate or incomplete MRI report that can delay critical treatment decisions [128]. Imaging centers should refer to the 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with MS to standardize scanning protocols in clinical practice [133]. The Multiple Sclerosis Association of America (MSAA) has an MRI Access Program to assist with the cost of cranial and cervical spine MRI scans for people who have MS or are being diagnosed [134]. Routine MRI scans are instrumental in timely identification of early MS disease activity and therefore should be adequately covered to allow for rapid and informed treatment decisions made by HCPs and patients.
Prescriptive insurance policies undermine the therapeutic alliance between patients and HCPs and can lead to treatment failure and delays in appropriate care, resulting in poor adherence, patient anxiety, and worsening MS disease activity and disability [124, 127]. As an alternative, early and unrestricted access to HETs and diagnostic and monitoring tests can provide freedom of choice for clinicians, optimize outcomes for patients, and potentially reduce payer and societal costs [10]. The latter approach is in line with a therapeutic strategy that recommends making all DMTs, including HETs, available to people with relapsing forms of MS to identify the optimal treatment for each person based on perceived efficacy or tolerability rather than cost [7]. Until this becomes the norm, patients and clinicians may have to spend considerable time petitioning insurance companies to obtain the appropriate medication [124, 135].
HCP- and service-related factors
The importance of preserving neurological function in people with MS by reducing delays at all stages of the care pipeline has been emphasized in a policy report by international MS experts [7]. However, delays commonly occur between MS symptom onset and confirmatory diagnosis by a specialist neurologist, arising due to administrative issues and poor awareness of MS symptoms among HCPs, patients, and their families [7]. National bodies, patient advocacy groups/associations, and/or professional bodies should aim to educate primary care physicians on referring suspected cases of MS promptly to a neurologist (preferably an MS specialist) to expedite appropriate care [7]. Referral delays may be exacerbated by neurologist hesitancy caused in part by the limited clinical guidance on early HET use and safety concerns [7, 8]. Familiarity with established DMTs is an influential factor and can result in treatment inertia (reported in up to 70% of neurologists) and delays switching from an established to a newer therapy, especially among non-MS neurologists [127, 136]. In the US, HCPs might be even more risk averse than patients due to different perspectives and clinical experience regarding potentially life-threatening safety risks, which can encourage more defensive medical practices out of concern for legal liability [137, 138]. Updated long-term safety profiles, which can be communicated through continuing medical education and peer-to-peer events [10, 139], will be critical for managing perceptions of HCPs and non-specialist neurologists in this regard.
Another reason for HCP hesitancy around early HET is the variance in vaccine response highlighted in the wake of the coronavirus disease 2019 (COVID-19) pandemic. The ability of anti-CD20 HETs to deplete B lymphocytes and potentially cause hypogammaglobulinemia raised concerns about whether HET-treated patients could achieve the appropriate immune response to a COVID-19 vaccine [3, 140]. Ocrelizumab has even been associated with a more severe course of COVID-19 in a real-world analysis [141]. However, there have been several reports of patients receiving anti-CD20s for MS being able to mount robust antigen-specific CD4 + and CD8 + T-cell responses to messenger RNA COVID-19 vaccines, even with impaired humoral responses [142–145]. Moreover, the DMT risks associated with COVID-19 may have been overestimated in earlier studies due to confounders [146], and HET effects on COVID-19 severity or infection risk vary according to patient population and treatment [147, 148]. Improving education about vaccine efficacy and the benefit-risk ratio of HETs regarding infection risk can help to address HCP concerns. A multidisciplinary approach to early treatment, involving MS specialist nurse navigators and pharmacists serving as accessible resources, can support the shared decision-making process and achieve more well-informed patient care [149, 150]. There may also be a call for involving infectious disease experts to manage timely risk mitigation in vulnerable patients.
For HCPs, selecting among many different DMTs can become even more challenging with time and financial constraints in clinical settings [127]. MS specialists must have sufficient time in the clinic to adequately educate patients on early treatment options [7]. Improving the quality and efficiency of decision-making in clinical practice can be assisted by comprehensive treatment algorithms using prognostic factors to identify higher-risk patients who would benefit the most from early HET [24, 151]. The eventual goal would be a personalized treatment approach encompassing prognostic factors (clinical, paraclinical, environmental, and demographic), patient-related factors (e.g., comorbidities, family planning, and level of risk aversion), and drug-related factors (i.e., safety, cost, and treatment sequencing options) [24]. Involving specialty pharmacists in the medication management of patients with MS may also relieve some pressure while optimizing patient care [152].
Additional systemic factors, such as poor continuity of care outside of MS clinical centers, long wait times to access secondary care, and inherent limitations in the ability of conventional MRIs to capture cortical lesions and regional atrophy, create problems for obtaining appropriate health care [150, 153, 154]. There may also be practicable challenges, as most HETs (alemtuzumab, natalizumab, ocrelizumab, and ublituximab) require intravenous administration at specialized infusion centers or tertiary hospitals [26, 29, 155]. Ofatumumab is an exception and can be self-administered by subcutaneous injection without the need for an infusion [156]. Access to infusion sites can have the additional benefit of promoting adherence [155]. An international panel of MS specialists and multidisciplinary reviewers recommend offering infusible therapy within 4 weeks following the patient’s agreement to initiate treatment, with an aspirational goal of initiating the DMT within 7 days [157]. To achieve this, resources at infusion sites should be strategically increased and/or expanded to additional locations and times to alleviate resource demand [155]. Expanding access to laboratory and vaccination centers would also expedite necessary screening and vaccinations prior to starting DMT.
Overcoming issues of hesitancy regarding early HET could be largely ameliorated by a consensus statement from national neurological and MS associations in the US. With this in place, expanding the future MS workforce by providing more educational and research grants in neurology and neuroimmunology could help to ensure that receptivity to the early HET approach is sustained over the long-term.
Patient-related factors
Different experiences in patients’ engagement with health care resources can influence their treatment decisions. First, health care resource utilization is lower among patients from underserved populations who are at high risk for disability, such as those from lower socioeconomic and minority racial/ethnic backgrounds [158–161]. Inequalities in access to health care services for people with MS are worse for men, older patients, lower socioeconomic groups/least educated, non-White (including African American and Hispanic), those with mental health problems, and those residing in rural areas [162–164]. People with MS who can see neurologists are more likely to receive DMTs and access specialists than those who see other providers, but the probability of seeing a neurologist is significantly lower for people who do not have health insurance, are poor, are living in rural areas, are African American/Hispanic, or have worse disability [158, 165, 166]. Access is further limited by the poor geographic distribution of MS centers across the US providing subspecialty care [166]. Both Black and Hispanic patients with MS frequently initiate low- and moderate-efficacy DMTs despite a greater risk of developing a severe disease course with greater disability [167, 168].
Second, even patients who are familiar with a neurologist or other HCP may not sufficiently learn about DMTs during routine consultations [169]. For example, studies have found that patients strongly favored medications that could improve symptoms, despite this not being the primary effect of DMTs [169]. Some patients have reported difficulties in finding relevant information about DMTs on the internet that does not require a high education level [170]. Comorbidities such as MS-related cognitive impairment, depression, fatigue, or anxiety may then contribute to poor understanding [169]. Furthermore, maintaining adherence can be a challenge for most MS medications, with reported rates of DMT adherence ranging from 52 to 92.8% [171]. Poor adherence has been linked to worse disease outcomes and increased costs [118] and may make an effective DMT appear ineffective, leading to unnecessary therapy changes [128].
Patients could be empowered to make fully informed decisions on their initial DMT by increasing access to MS centers and improving the quality of HCP-patient interactions [7, 127, 150]. A tailored, multifaceted approach to DMT selection may help to identify patients, especially those in underserved populations, who would most benefit from HET to maximize their treatment outcomes [168]. Low-income minorities can be encouraged to initiate and adhere to treatment through adequate education about the disease course, treatment goals and options, and community resources [159]. The MSAA and National Multiple Sclerosis Society provide information and resources in Spanish to serve the rapidly growing US Hispanic population [172, 173]. In response to inequalities in MS care among underserved populations, ongoing US-based registries for Hispanic and African American populations with MS have been established to collect incidence and prevalence data, educate patients and HCPs, and learn about issues affecting access to MS care [164, 174].
HCPs have a responsibility to support patients in understanding their options during clinical discussions. They should demystify important topics, such as the benefits of early treatment; information on new DMTs, without overemphasizing the perceived risks; and the consequences of suboptimal treatment [7, 127]. Counseling patients on DMT use around pregnancy may also support early HET use in those who feel inadequately informed about the implications on family planning [175, 176]. Patient engagement could benefit from the use of tools, such as decision aids, health coaching, and question prompts, along with providing personalized information, using motivational interviewing, and directing to useful resources [177]. Reliable sources of information on HETs include patient advocacy websites, such as the MSAA Ultimate MS Treatment Guide [178], MS neurologists’ curated platforms on social media [179, 180], and the MS Living Well website with its associated podcast [181], which educate hundreds of thousands of patients globally.
Shared decision-making between HCPs and patients is a key component to improving acceptance of, and adherence to, DMTs [56, 127]. It also allows patients to communicate their preferences, which may differ from those of HCPs [137, 138, 169]. For instance, route of administration is an important consideration for many patients [182]. Likewise, patient concerns about the long-term risks of early intervention with HETs, particularly infections and malignancy [8], may motivate preferences for DMTs with a perceived lower risk of significant side effects [169]. Others may prefer treatments with only moderate, but guaranteed benefits [169]. American Academy of Neurology guidelines recommend that HCPs take into consideration patient preferences around safety, administration route, medication frequency, monitoring, and lifestyle when deciding on an initial therapy [56]. Certain HETs could satisfy such patient requirements: both ocrelizumab (administered twice a year by intravenous infusion) and ofatumumab (self-administered once a month by subcutaneous injection) have demonstrated superior adherence and persistence to other non-HET injectable and oral DMTs [183–185]. Sharing all therapeutic options with patients, including those related to an early HET approach, encourages greater participation in their health care management—an outcome that could not only improve disease education and treatment satisfaction but also ultimately improve their long-term health outcomes.
Conclusions
Emerging evidence supports initiating early HET after an MS diagnosis to maximize patient outcomes. Implementing early HET in the US is hindered by various barriers that delay much-needed updates to best practices. Encouraging adoption of early HET strategies will be aided by long-term safety data to update clinical guidelines and mitigate safety concerns, improving patient and HCP education to empower shared decision-making for all patients regardless of background, and implementing policy changes that expand access to HETs at the local and national levels. Advocacy is needed at all levels—HCPs, insurers, and patient groups—to encourage reevaluation of current clinical guidelines and approval of first-line HET on insurance formularies. Doing so may be imperative to creating meaningful change in the US health care system and continually improving outcomes for people with MS.
Acknowledgements
Medical writing support was provided by Chloe Koulouris, PhD, of Envision Pharma, Inc., and was funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice (GPP) guidelines. Authors had full control of the content and made the final decision on all aspects of this article.
Author contributions
BAS: Conceptualization; Writing – original draft; Writing – review & editing. JF: Conceptualization; Writing – original draft; Writing – review & editing. HC-R: Conceptualization; Writing – original draft; Writing – review & editing.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by Novartis Pharmaceuticals Corporation. The authors received no honoraria related to the development of this publication.
Data availability
Not applicable.
Declarations
Conflicts of interest
BS has received research grant support from AbbVie, Biogen, Bristol Myers Squibb, Greenwich Biosciences, Novartis, Sanofi, and TG Therapeutics and consulting and/or speaking fees from Alexion, Biogen, Bristol Myers Squibb, Cionic, Cycle, EMD Serono, Genentech, Horizon, Janssen, Novartis, Octave Bioscience, Roche, Sanofi, Sandoz, and TG Therapeutics. JF has served on advisory boards for Bristol Myers Squibb, Genentech, Horizon, Novartis, and TG Therapeutics. HCR is a paid speaker for Biogen and Janssen, has served on advisory board for Genentech, and has received fellowship grant support from the National MS Society.
References
- 1.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol. 2018;31:233–243. doi: 10.1097/WCO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 4.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 5.Makhani N, Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17:515–521. doi: 10.1038/s41582-021-00519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cree BAC, Hollenbach JA, Bove R, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol. 2022;269:5382–5394. doi: 10.1007/s00415-022-11193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman L, Longbrake EE, Coyle PK, Hendin B, Vollmer T. High-efficacy therapies for treatment-naive individuals with relapsing-remitting multiple sclerosis. CNS Drugs. 2022;36:1285–1299. doi: 10.1007/s40263-022-00965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippi M, Danesi R, Derfuss T, et al. Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol. 2022;269:1670–1677. doi: 10.1007/s00415-021-10836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765–779. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt C, McGinley MP. Advances in the treatment of multiple sclerosis. Neurol Clin. 2021;39:21–33. doi: 10.1016/j.ncl.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76:536–541. doi: 10.1001/jamaneurol.2018.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 15.Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology. 2020;95:e1041–e1051. doi: 10.1212/WNL.0000000000010135. [DOI] [PubMed] [Google Scholar]
- 16.Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78:1197–1204. doi: 10.1001/jamaneurol.2021.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uher T, Krasensky J, Malpas C, et al. Evolution of brain volume loss rates in early stages of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e979. doi: 10.1212/NXI.0000000000000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS) [NCT03535298]. U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03535298. Accessed 18 January, 2024
- 19.ClinicalTrials.gov Traditional Versus Early Aggressive Therapy for Multiple Sclerosis Trial (TREAT-MS) [NCT03500328]. U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03500328. Accessed 18 January, 2024
- 20.Marques VD, Passos GRD, Mendes MF, et al. Brazilian consensus for the treatment of multiple sclerosis: Brazilian Academy of Neurology and Brazilian Committee on Treatment and Research in Multiple Sclerosis. Arq Neuropsiquiatr. 2018;76:539–554. doi: 10.1590/0004-282X20180078. [DOI] [PubMed] [Google Scholar]
- 21.Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15:273–279. doi: 10.1136/practneurol-2015-001139. [DOI] [PubMed] [Google Scholar]
- 22.Samjoo IA, Worthington E, Drudge C, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10:495–507. doi: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 23.Giovannoni G, Lang S, Wolff R, et al. A systematic review and mixed treatment comparison of pharmaceutical interventions for multiple sclerosis. Neurol Ther. 2020;9:359–374. doi: 10.1007/s40120-020-00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300. doi: 10.1038/s41582-019-0170-8. [DOI] [PubMed] [Google Scholar]
- 25.Lucchetta RC, Tonin FS, Borba HHL, et al. Disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. CNS Drugs. 2018;32:813–826. doi: 10.1007/s40263-018-0541-5. [DOI] [PubMed] [Google Scholar]
- 26.Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper) Ther Adv Neurol Disord. 2021;14:17562864211039648. doi: 10.1177/17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 28.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 29.Steinman L, Fox E, Hartung HP, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387:704–714. doi: 10.1056/NEJMoa2201904. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 31.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 32.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 33.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 34.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 35.Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- 36.Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18:1021–1033. doi: 10.1016/S1474-4422(19)30238-8. [DOI] [PubMed] [Google Scholar]
- 37.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18:1009–1020. doi: 10.1016/S1474-4422(19)30239-X. [DOI] [PubMed] [Google Scholar]
- 38.Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol. 2021;78:558–567. doi: 10.1001/jamaneurol.2021.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 40.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 41.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 42.Naismith RT, Wolinsky JS, Wundes A, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: Interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2020;26:1729–1739. doi: 10.1177/1352458519881761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 44.Confavreux C, O'Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 45.Khan O, Rieckmann P, Boyko A, et al. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol. 2013;73:705–713. doi: 10.1002/ana.23938. [DOI] [PubMed] [Google Scholar]
- 46.Ebers GC, PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. doi: 10.1016/S0140-6736(98)03334-0. [DOI] [PubMed] [Google Scholar]
- 47.Ontaneda D, Tallantyre EC, Raza PC, et al. Determining the effectiveness of early intensive versus escalation approaches for the treatment of relapsing-remitting multiple sclerosis: the DELIVER-MS study protocol. Contemp Clin Trials. 2020;95:106009. doi: 10.1016/j.cct.2020.106009. [DOI] [PubMed] [Google Scholar]
- 48.Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol. 2019;32:365–377. doi: 10.1097/WCO.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 49.Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e636. doi: 10.1212/NXI.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389:1347–1356. doi: 10.1016/S0140-6736(16)32388-1. [DOI] [PubMed] [Google Scholar]
- 51.Rommer PS, Milo R, Han MH, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bermel RA, Weinstock-Guttman B, Bourdette D, et al. Intramuscular interferon beta-1a therapy in patients with relapsing-remitting multiple sclerosis: a 15-year follow-up study. Mult Scler. 2010;16:588–596. doi: 10.1177/1352458509360549. [DOI] [PubMed] [Google Scholar]
- 53.Rieckmann P, Zivadinov R, Boyko A, et al. Long-term efficacy and safety of three times weekly dosing regimen of glatiramer acetate in relapsing multiple sclerosis patients: seven-year results of the Glatiramer Acetate Low-frequency Administration (GALA) open-label extension study. Mult Scler J Exp Transl Clin. 2021;7:20552173211061550. doi: 10.1177/20552173211061550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller AE, Olsson TP, Wolinsky JS, et al. Long-term safety and efficacy of teriflunomide in patients with relapsing multiple sclerosis: results from the TOWER extension study. Mult Scler Relat Disord. 2020;46:102438. doi: 10.1016/j.msard.2020.102438. [DOI] [PubMed] [Google Scholar]
- 55.CMSC DMT Guideline Writing Group (2019) CMSC practical guidelines for the selection of disease-modifying therapies in multiple sclerosis. https://cmscscholar.org/cmsc-practical-guidelines-for-the-selection-of-disease-modifying-therapies-in-ms/. Accessed 20 January, 2023
- 56.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 57.Kalincik T, Jokubaitis V, Izquierdo G, et al. Comparative effectiveness of glatiramer acetate and interferon beta formulations in relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:1159–1171. doi: 10.1177/1352458514559865. [DOI] [PubMed] [Google Scholar]
- 58.IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86:920–930. doi: 10.1212/WNL.0000000000002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kappos L, Kuhle J, Multanen J, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015;86:1202–1207. doi: 10.1136/jnnp-2014-310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otero-Romero S, Sánchez-Montalvá A, Vidal-Jordana A. Assessing and mitigating risk of infection in patients with multiple sclerosis on disease modifying treatment. Expert Rev Clin Immunol. 2021;17:285–300. doi: 10.1080/1744666X.2021.1886924. [DOI] [PubMed] [Google Scholar]
- 62.Genzyme S. Aubagio [package insert] Cambridge, MA: Genzyme Corporation; 2022. [Google Scholar]
- 63.Andersen JB, Wandall-Holm MF, Magyari M. Pregnancy outcomes following maternal or paternal exposure to teriflunomide in the Danish MS population. Mult Scler Relat Disord. 2022;59:103529. doi: 10.1016/j.msard.2022.103529. [DOI] [PubMed] [Google Scholar]
- 64.Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther. 2014;3:133–138. doi: 10.1007/s40120-014-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: clinical study data and 5 years of post-marketing experience. Mult Scler. 2020;26:829–836. doi: 10.1177/1352458519843055. [DOI] [PubMed] [Google Scholar]
- 66.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 67.Cree BAC, Goldman MD, Corboy JR, et al. Efficacy and safety of 2 fingolimod doses vs glatiramer acetate for the treatment of patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2020;78:48–60. doi: 10.1001/jamaneurol.2020.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merck . Mavenclad [package insert] Rockland, MA: EMD Serono Inc; 2022. [Google Scholar]
- 69.Novartis . Gilenya [package insert] East Hanover, NJ: Novartis AG; 2019. [Google Scholar]
- 70.Janssen Pharmaceuticals Inc . Ponvory [package insert] Titusville, NJ: Janssen Pharmaceuticals Inc; 2021. [Google Scholar]
- 71.Corporation C. Zeposia [package insert] Summit, NJ: Celgene Corporation; 2022. [Google Scholar]
- 72.Novartis . Mayzent [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2023. [Google Scholar]
- 73.Young CA, Mills R, Rog D, et al. Quality of life in multiple sclerosis is dominated by fatigue, disability and self-efficacy. J Neurol Sci. 2021;426:117437. doi: 10.1016/j.jns.2021.117437. [DOI] [PubMed] [Google Scholar]
- 74.Gil-González I, Martín-Rodríguez A, Conrad R, Pérez-San-Gregorio MÁ. Quality of life in adults with multiple sclerosis: a systematic review. BMJ Open. 2020;10:e041249. doi: 10.1136/bmjopen-2020-041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dymecka J, Gerymski R, Tataruch R, Bidzan M. Sense of coherence and health-related quality of life in patients with multiple sclerosis: the role of physical and neurological disability. J Clin Med. 2022;11:1716. doi: 10.3390/jcm11061716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23:1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147–3161. doi: 10.1093/brain/awac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the 'real MS'. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi: 10.1177/17562864211066751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hausser-Kinzel S, Weber MS. The role of B cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front Immunol. 2019;10:201. doi: 10.3389/fimmu.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ransohoff RM. Multiple sclerosis: role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol. 2023;44:266–275. doi: 10.1016/j.it.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132–1140. doi: 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256:405–415. doi: 10.1007/s00415-009-0093-1. [DOI] [PubMed] [Google Scholar]
- 83.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 84.Gärtner J, Hauser SL, Bar-Or A, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult Scler. 2022;28:1562–1575. doi: 10.1177/13524585221078825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321:175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iaffaldano P, Lucisano G, Caputo F, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. doi: 10.1177/17562864211019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simonsen CS, Flemmen HØ, Broch L, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. doi: 10.3389/fneur.2021.693017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hrnciarova T, Drahota J, Spelman T, et al. Does initial high efficacy therapy in multiple sclerosis surpass escalation treatment strategy? A comparison of patients with relapsing-remitting multiple sclerosis in the Czech and Swedish national multiple sclerosis registries. Mult Scler Relat Disord. 2023;76:104803. doi: 10.1016/j.msard.2023.104803. [DOI] [PubMed] [Google Scholar]
- 89.Pipek LZ, Mahler JV, Nascimento RFV, et al. Cost, efficacy, and safety comparison between early intensive and escalating strategies for multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2023;71:104581. doi: 10.1016/j.msard.2023.104581. [DOI] [PubMed] [Google Scholar]
- 90.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. doi: 10.3389/fneur.2017.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chalmer TA, Baggesen LM, Nørgaard M, et al. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol. 2018;25:1262–e1110. doi: 10.1111/ene.13692. [DOI] [PubMed] [Google Scholar]
- 92.Saidha S, Bell J, Harold S, et al. Systematic literature review of immunoglobulin trends for anti-CD20 monoclonal antibodies in multiple sclerosis. Neurol Sci. 2023;44:1515–1532. doi: 10.1007/s10072-022-06582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mears V, Jakubecz C, Seeco C, et al. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J Neuroimmunol. 2023;377:578066. doi: 10.1016/j.jneuroim.2023.578066. [DOI] [PubMed] [Google Scholar]
- 94.Oksbjerg NR, Nielsen SD, Blinkenberg M, Magyari M, Sellebjerg F. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult Scler Relat Disord. 2021;52:102988. doi: 10.1016/j.msard.2021.102988. [DOI] [PubMed] [Google Scholar]
- 95.Cohen JA, Hauser SL, Cross AH, et al. Five-year safety of ofatumumab in people living with relapsing multiple sclerosis. Neurology. 2023;100:2942. doi: 10.1212/WNL.0000000000202906. [DOI] [Google Scholar]
- 96.Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord. 2017;10:343–359. doi: 10.1177/1756285617722706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hauser SL, Cross AH, Winthrop K, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28:1576–1590. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hauser SL, Kappos L, Arnold DL, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 2020;95:e1854–e1867. doi: 10.1212/WNL.0000000000010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP) J Neurol Neurosurg Psychiatry. 2020;91:660–668. doi: 10.1136/jnnp-2019-322326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Biogen Inc. Tysabri [package insert] Cambridge, MA: Biogen Inc.; 2021. [Google Scholar]
- 101.Ho PR, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925–933. doi: 10.1016/S1474-4422(17)30282-X. [DOI] [PubMed] [Google Scholar]
- 102.Ziemssen T, Bass AD, Berkovich R, et al. Efficacy and safety of alemtuzumab through 9 years of follow-up in patients with highly active disease: post hoc analysis of CARE-MS I and II patients in the TOPAZ extension study. CNS Drugs. 2020;34:973–988. doi: 10.1007/s40263-020-00749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Genzyme Corp. (2021) Lemtrada [package insert]. vol 2021. Cambridge, MA
- 104.Morrow SA, Clift F, Devonshire V, et al. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult Scler Relat Disord. 2022;65:103995. doi: 10.1016/j.msard.2022.103995. [DOI] [PubMed] [Google Scholar]
- 105.Schriefer D, Haase R, Ness NH, Ziemssen T. Cost of illness in multiple sclerosis by disease characteristics – a review of reviews. Expert Rev Pharmacoecon Outcomes Res. 2022;22:177–195. doi: 10.1080/14737167.2022.1987218. [DOI] [PubMed] [Google Scholar]
- 106.Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89:844–850. doi: 10.1136/jnnp-2017-317509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bebo B, Cintina I, LaRocca N, et al. The economic burden of multiple sclerosis in the United States: estimate of direct and indirect costs. Neurology. 2022;98:e1810–e1817. doi: 10.1212/WNL.0000000000200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kobelt G, Langdon D, Jönsson L. The effect of self-assessed fatigue and subjective cognitive impairment on work capacity: the case of multiple sclerosis. Mult Scler. 2019;25:740–749. doi: 10.1177/1352458518769837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.National Multiple Sclerosis Society (2023) Fatigue. https://www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms/Fatigue. Accessed 15 February, 2023
- 110.Lakin L, Davis BE, Binns CC, Currie KM, Rensel MR. Comprehensive approach to management of multiple sclerosis: addressing invisible symptoms-a narrative review. Neurol Ther. 2021;10:75–98. doi: 10.1007/s40120-021-00239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penner IK, McDougall F, Brown TM, et al. Exploring the impact of fatigue in progressive multiple sclerosis: a mixed-methods analysis. Mult Scler Relat Disord. 2020;43:102207. doi: 10.1016/j.msard.2020.102207. [DOI] [PubMed] [Google Scholar]
- 112.van Egmond EEA, van Gorp DAM, Jongen PJ, et al. Self-reported work productivity in people with multiple sclerosis and its association with mental and physical health. Disabil Rehabil. 2022;44:7096–7105. doi: 10.1080/09638288.2021.1981468. [DOI] [PubMed] [Google Scholar]
- 113.Sainz de la Maza S, Maurino J, Borges M, et al. Measuring productivity loss in early relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2022;58:103398. doi: 10.1016/j.msard.2021.103398. [DOI] [PubMed] [Google Scholar]
- 114.Batcheller L, Baker D. Cost of disease modifying therapies for multiple sclerosis: is front-loading the answer? J Neurol Sci. 2019;404:19–28. doi: 10.1016/j.jns.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Koeditz D, Frensch J, Bierbaum M, et al. Comparing the long-term clinical and economic impact of ofatumumab versus dimethyl fumarate and glatiramer acetate in patients with relapsing multiple sclerosis: a cost-consequence analysis from a societal perspective in Germany. Mult Scler J Exp Transl Clin. 2022;8:20552173221085741. doi: 10.1177/20552173221085741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ness NH, Schriefer D, Haase R, et al. Differentiating societal costs of disability worsening in multiple sclerosis. J Neurol. 2020;267:1035–1042. doi: 10.1007/s00415-019-09676-4. [DOI] [PubMed] [Google Scholar]
- 117.Ness NH, Schriefer D, Haase R, Ettle B, Ziemssen T. Real-world evidence on the societal economic relapse costs in patients with multiple sclerosis. Pharmacoeconomics. 2020;38:883–892. doi: 10.1007/s40273-020-00917-3. [DOI] [PubMed] [Google Scholar]
- 118.Lizán L, Comellas M, Paz S, et al. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–1664. doi: 10.2147/PPA.S67253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geiger C, Sheinson D, To T, Jones D, Bonine N. ACTRIMS Forum 2022–P330. Characteristics of newly diagnosed MS patients initiating high, moderate, and low-efficacy disease modifying therapies as first-line treatment. Mult Scler. 2022;28:20–214. doi: 10.1177/13524585221094743. [DOI] [Google Scholar]
- 120.Henderson M, Horton DB, Bhise V, et al. Initiation patterns of disease-modifying therapies for multiple sclerosis among US adults and children, 2001 through 2020. JAMA Neurol. 2023;80:860–867. doi: 10.1001/jamaneurol.2023.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mateen FJ, Trápaga Hacker C. Perceptions of people with multiple sclerosis on social determinants of health: mixed methods. Mult Scler Relat Disord. 2023;80:105089. doi: 10.1016/j.msard.2023.105089. [DOI] [PubMed] [Google Scholar]
- 122.Moghavem N, Castañeda GDR, Chatfield AJ, Amezcua L. The impact of medical insurance on health care access and quality for people with multiple sclerosis in the United States: a scoping review. Mult Scler. 2023;12:13524585231197275. doi: 10.1177/13524585231197275. [DOI] [PubMed] [Google Scholar]
- 123.Seifer G, Arun T, Capela C, et al. Influence of physicians' risk perception on switching treatments between high-efficacy and non-high-efficacy disease-modifying therapies in multiple sclerosis. Mult Scler Relat Disord. 2023;76:104770. doi: 10.1016/j.msard.2023.104770. [DOI] [PubMed] [Google Scholar]
- 124.Bourdette DN, Hartung DM, Whitham RH. Practices of US health insurance companies concerning MS therapies interfere with shared decision-making and harm patients. Neurol Clin Pract. 2016;6:177–182. doi: 10.1212/CPJ.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mizell R. The impact of insurance restrictions in newly diagnosed individuals with multiple sclerosis. Int J MS Care. 2024;26:17–21. doi: 10.7224/1537-2073.2022-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang G, Marrie RA, Salter AR, et al. Health insurance affects the use of disease-modifying therapy in multiple sclerosis. Neurology. 2016;87:365–374. doi: 10.1212/WNL.0000000000002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rieckmann P, Centonze D, Elovaara I et al; Members of the MS in the 21st Century Steering Group (2018) Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord 19:153–160. 10.1016/j.msard.2017.11.013 [DOI] [PubMed]
- 128.Weinstein DR, Owens GM, Gandhi A. Multiple sclerosis: systemic challenges to cost-effective care. Am Health Drug Benefits. 2022;15:13–20. [PMC free article] [PubMed] [Google Scholar]
- 129.Hartung DM. Economics of multiple sclerosis disease-modifying therapies in the USA. Curr Neurol Neurosci Rep. 2021;21:28. doi: 10.1007/s11910-021-01118-x. [DOI] [PubMed] [Google Scholar]
- 130.Hartung DM, Johnston KA, Bourdette DN, Chen R, Tseng CW. Closing the Part D coverage gap and out-of-pocket costs for multiple sclerosis drugs. Neurol Clin Pract. 2021;11:298–303. doi: 10.1212/CPJ.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.National Multiple Sclerosis Society (2022) Make MS medications accessible. https://www.nationalmssociety.org/Treating-MS/Medications/Make-MS-Medications-Accessible. Accessed 6 October, 2022
- 132.New Choice Health. What can affect the cost of an MRI? https://www.newchoicehealth.com/mri/cost. Accessed 16 February, 2023
- 133.Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20:653–670. doi: 10.1016/S1474-4422(21)00095-8. [DOI] [PubMed] [Google Scholar]
- 134.Multiple Sclerosis Association of America (2022) MRI access program. https://mymsaa.org/msaa-help/mri/. Accessed 16 February, 2022
- 135.Simacek KF, Ko JJ, Moreton D, et al. The impact of disease-modifying therapy access barriers on people with multiple sclerosis: mixed-methods study. J Med Internet Res. 2018;20:e11168. doi: 10.2196/11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saposnik G, Sempere AP, Prefasi D, et al. Decision-making in multiple sclerosis: the role of aversion to ambiguity for therapeutic inertia among neurologists (DIScUTIR MS) Front Neurol. 2017;8:65. doi: 10.3389/fneur.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar J, Cambron-Mellott MJ, Tencer T, et al. Patient and neurologist preferences in the United States for relapsing-remitting multiple sclerosis treatments: findings from a discrete choice experiment. Patient Prefer Adherence. 2021;15:1515–1527. doi: 10.2147/PPA.S306498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kremer IEH, Evers S, Jongen PJ, Hiligsmann M. Comparison of preferences of healthcare professionals and MS patients for attributes of disease-modifying drugs: a best-worst scaling. Health Expect. 2018;21:171–180. doi: 10.1111/hex.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Péloquin S, Schmierer K, Leist TP, et al. Challenges in multiple sclerosis care: results from an international mixed-methods study. Mult Scler Relat Disord. 2021;50:102854. doi: 10.1016/j.msard.2021.102854. [DOI] [PubMed] [Google Scholar]
- 140.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simpson-Yap S, Pirmani A, Kalincik T, et al. Updated results of the COVID-19 in MS Global Data Sharing Initiative: anti-CD20 and other risk factors associated with COVID-19 severity. Neurol Neuroimmunol Neuroinflamm. 2022;9:e200021. doi: 10.1212/NXI.0000000000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim E, Haag A, Nguyen J, Kesselman MM, Demory Beckler M. Vaccination of multiple sclerosis patients during the COVID-19 era: novel insights into vaccine safety and immunogenicity. Mult Scler Relat Disord. 2022;67:104172. doi: 10.1016/j.msard.2022.104172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madelon N, Lauper K, Breville G, et al. Robust T-cell responses in anti-CD20-treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. 2022;75:e1037–e1045. doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alfonso-Dunn R, Lin J, Kirschner V, et al. Strong T-cell activation in response to COVID-19 vaccination in multiple sclerosis patients receiving B-cell depleting therapies. Front Immunol. 2022;13:926318. doi: 10.3389/fimmu.2022.926318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Longinetti E, Bower H, McKay KA, et al. COVID-19 clinical outcomes and DMT of MS patients and population-based controls. Ann Clin Transl Neurol. 2022;9:1449–1458. doi: 10.1002/acn3.51646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Januel E, Hajage D, Labauge P, et al. Association between anti-CD20 therapies and COVID-19 severity among patients with relapsing-remitting and progressive multiple sclerosis. JAMA Netw Open. 2023;6:e2319766. doi: 10.1001/jamanetworkopen.2023.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith TE, Madhavan M, Gratch D, et al. Risk of COVID-19 infection and severe disease in MS patients on different disease-modifying therapies. Mult Scler Relat Disord. 2022;60:103735. doi: 10.1016/j.msard.2022.103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bainbridge J, Barnhart R, Fuller R, et al. The role of clinical pharmacists in patient-centric comprehensive multiple sclerosis care. Int J MS Care. 2024;26:1–7. doi: 10.7224/1537-2073.2022-051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mayo CD, Farzam-Kia N, Ghahari S. Identifying barriers to and facilitators of health service access encountered by individuals with multiple sclerosis. Int J MS Care. 2021;23:37–44. doi: 10.7224/1537-2073.2020-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tintore M, Rovira A, Río J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138:1863–1874. doi: 10.1093/brain/awv105. [DOI] [PubMed] [Google Scholar]
- 152.Schultz J, Weber C, Kamholz J. Letter to the editor: the emerging role of pharmacists in the multidisciplinary care of patients with multiple sclerosis. Int J MS Care. 2016;18:219–220. doi: 10.7224/1537-2073.2015-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Combes AJE, Clarke MA, O'Grady KP, Schilling KG, Smith SA. Advanced spinal cord MRI in multiple sclerosis: current techniques and future directions. Neuroimage Clin. 2022;36:103244. doi: 10.1016/j.nicl.2022.103244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11:597–606. doi: 10.1038/nrneurol.2015.157. [DOI] [PubMed] [Google Scholar]
- 155.Rath L, Campagna MP, Stankovich J, et al. Patient preferences for time and location of infusible therapies in multiple sclerosis and neuroimmunologic disorders. Int J MS Care. 2021;23:114–118. doi: 10.7224/1537-2073.2020-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Novartis . Kesimpta [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2022. [Google Scholar]
- 157.Hobart J, Bowen A, Pepper G, et al. International consensus on quality standards for brain health-focused care in multiple sclerosis. Mult Scler. 2019;25:1809–1818. doi: 10.1177/1352458518809326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saadi A, Himmelstein DU, Woolhandler S, Mejia NI. Racial disparities in neurologic health care access and utilization in the United States. Neurology. 2017;88:2268–2275. doi: 10.1212/WNL.0000000000004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shabas D, Heffner M. Multiple sclerosis management for low-income minorities. Mult Scler. 2005;11:635–640. doi: 10.1191/1352458505ms1215oa. [DOI] [PubMed] [Google Scholar]
- 160.Amezcua L, Smith JB, Gonzales EG, Haraszti S, Langer-Gould A. Race, ethnicity, and cognition in persons newly diagnosed with multiple sclerosis. Neurology. 2020;94:e1548–e1556. doi: 10.1212/WNL.0000000000009210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brayo P, Kimbrough D (2021) Multiple sclerosis in the Black population of the United States. https://practicalneurology.com/articles/2021-feb/multiple-sclerosis-in-the-black-population-of-the-united-states. Accessed 11 January, 2021
- 162.Roddam H, Rog D, Janssen J, et al. Inequalities in access to health and social care among adults with multiple sclerosis: a scoping review of the literature. Mult Scler Relat Disord. 2019;28:290–304. doi: 10.1016/j.msard.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 163.Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611–620. doi: 10.1016/j.puhe.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 164.Amezcua L, Oksenberg JR, McCauley JL. MS in self-identified Hispanic/Latino individuals living in the US. Mult Scler J Exp Transl Clin. 2017;3:2055217317725103. doi: 10.1177/2055217317725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Minden SL, Hoaglin DC, Hadden L, et al. Access to and utilization of neurologists by people with multiple sclerosis. Neurology. 2008;70:1141–1149. doi: 10.1212/01.wnl.0000306411.46934.ef. [DOI] [PubMed] [Google Scholar]
- 166.McGinley MP, Harvey T, Lopez R, Ontaneda D, Buchalter RB. Geographic disparities in access to neurologists and multiple sclerosis care in the United States. Neurology. 2024;102:e207916. doi: 10.1212/WNL.0000000000207916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Geiger CK, Sheinson D, To TM, Jones D, Bonine NG. Treatment patterns by race and ethnicity in newly diagnosed persons with multiple sclerosis. Drugs Real World Outcomes. 2023 doi: 10.1007/s40801-023-00387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pérez CA, Lincoln JA. Racial and ethnic disparities in treatment response and tolerability in multiple sclerosis: a comparative study. Mult Scler Relat Disord. 2021;56:103248. doi: 10.1016/j.msard.2021.103248. [DOI] [PubMed] [Google Scholar]
- 169.Reen GK, Silber E, Langdon DW. Multiple sclerosis patients' understanding and preferences for risks and benefits of disease-modifying drugs: a systematic review. J Neurol Sci. 2017;375:107–122. doi: 10.1016/j.jns.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 170.Moccia M, Carotenuto A, Massarelli M, Lanzillo R, Brescia Morra V. Can people with multiple sclerosis actually understand what they read in the internet age? J Clin Neurosci. 2016;25:167–168. doi: 10.1016/j.jocn.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 171.Washington F, Langdon D. Factors affecting adherence to disease-modifying therapies in multiple sclerosis: systematic review. J Neurol. 2022;269:1861–1872. doi: 10.1007/s00415-021-10850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Multiple Sclerosis Association of America (2023) Información en Español. https://mymsaa.org/ms-information/spanish. Accessed 13 January, 2023
- 173.National Multiple Sclerosis Society (2023) Información y recursos sobre la esclerosis múltiple. https://www.nationalmssociety.org/Resources-Support/Library-Education-Programs/Informacion-en-Espanol. Accessed 15 February, 2023
- 174.Okai AF, Howard AM, Williams MJ, et al. Advancing care and outcomes for African American patients with multiple sclerosis. Neurology. 2022;98:1015–1020. doi: 10.1212/WNL.0000000000200791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of women with multiple sclerosis planning pregnancy. Curr Treat Options Neurol. 2021;23:11. doi: 10.1007/s11940-021-00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rasmussen PV, Magyari M, Moberg JY, et al. Patient awareness about family planning represents a major knowledge gap in multiple sclerosis. Mult Scler Relat Disord. 2018;24:129–134. doi: 10.1016/j.msard.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 177.Navigating MS Steering Committee (2017) Optimizing communication in the evolving multiple sclerosis benefit:risk landscape. https://mymsaa.org/PDFs/navigating_ms.pdf. Accessed 15 February, 2023
- 178.Multiple Sclerosis Association of America (2023) MSAA ultimate MS treatment guide. https://mymsaa.org/ms-information/treatments/guide/. Accessed 13 April, 2023
- 179.Beaber B (2023) Home [YouTube Channel]. YouTube. https://www.youtube.com/@DrBrandonBeaber. Accessed 13 April, 2023
- 180.Boster A (2023) Home [YouTube Channel]. YouTube. https://www.youtube.com/@AaronBosterMD. Accessed 13 April, 2023
- 181.MS Living Well (2023) MS Living Well. https://www.mslivingwell.org/. Accessed 13 April, 2023
- 182.Bottomley C, Lloyd A, Bennett G, Adlard N. A discrete choice experiment to determine UK patient preference for attributes of disease modifying treatments in multiple sclerosis. J Med Econ. 2017;20:863–870. doi: 10.1080/13696998.2017.1336099. [DOI] [PubMed] [Google Scholar]
- 183.Engmann NJ, Sheinson D, Bawa K, Ng CD, Pardo G. Persistence and adherence to ocrelizumab compared with other disease-modifying therapies for multiple sclerosis in U.S. commercial claims data. J Manag Care Spec Pharm. 2021;27:639–649. doi: 10.18553/jmcp.2021.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gorritz M, Chen C, Tuly R et al (2023) Real-world persistence of ofatumumab vs. oral disease modifying therapies in patients with multiple sclerosis. Presented at the Consoritum of Multiple Sclerosis Centers Annual Meeting, 31 May-3 June, Aurora, CO
- 185.Gorritz M, Chen C, Tuly R et al (2023) Real-world persistence and adherence of ofatumumab versus platform self-injectable disease modifying therapies in patients with multiple sclerosis. Presented at the Consortium of Multiple Sclerosis Centers Annual Meeting, 31 May–3 June, Aurora, CO
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.