Abstract
Introduction
Long-term (1-year) fremanezumab treatment proved to be effective, safe, and well tolerated in individuals with migraine and < 2 medication clusters in a randomized controlled trial (RCT). We aimed to assess real-world evidence (RWE), long-term effectiveness, tolerability, and safety of fremanezumab in people with high-frequency episodic migraine (HFEM) or chronic migraine (CM) with > 3 treatment failures and various comorbidities.
Methods
A 48-week, prospective, multicenter (n = 26), cohort study assessed fremanezumab’s effectiveness, safety, and tolerability in consecutive adults with HFEM or CM with > 3 treatment failures. Primary endpoint was variation from baseline in monthly migraine days (MMD) in HFEM and monthly headache days (MHD) in CM at weeks 45–48. Secondary endpoints were changes in monthly analgesic medications, Numerical Rating Scale (NRS), Headache Impact Test (HIT-6), and the Migraine Disability Assessment Scale (MIDAS) scores and ≥ 50%, ≥ 75%, and 100% responder rates.
Results
Of 533 participants who had received ≥ 1 fremanezumab dose, 130 were treated for ≥ 48 weeks and considered for effectiveness analysis. No participant missed any treatment dosage every other consecutive month during the 12-month period. Primary endpoint: fremanezumab significantly (p < 0.001) reduced both MMD (− 6.4) in HFEM and MHD (− 14.5) in CM. Secondary endpoints: a significant reduction (p < 0.001) was observed in monthly analgesic medications (HFEM − 6.0; CM −16.5), NRS (HFEM − 3.4; CM − 3.4), HIT-6 (HFEM − 16.9; CM − 17.9) and MIDAS score (HFEM − 50.4; CM − 76.6). The ≥ 50%, ≥ 75%, and 100% response rates to fremanezumab were 75.5%, 36.7%, and 2% in HFEM and 71.6%, 44.4%, and 3.7% in CM. Corresponding response rates were 60.5%, 37.2%, and 2.3% in individuals with psychiatric comorbidities, 74.2%, 50%, and 4.8% in CM with medication overuse, and 60.9%, 39.1%, and 4.3% in CM with medication overuse and psychiatric comorbidities. Mild and transient treatment-emergent adverse events occurred in 7.8% of the participants. No subject discontinued the treatment for any reason.
Conclusion
This RWE study documents that long-term fremanezumab treatment is highly effective and remarkably well tolerated in subjects with HFEM or CM with multiple (> 3) therapeutic failures, even in the presence of concomitant medication overuse, psychiatric comorbidities, or both. The effectiveness-to-tolerability ratio appears to be better in RWE than in RCTs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00591-z.
Keywords: Fremanezumab, Migraine treatment, CGRP monoclonal antibody, Real-world, Long-term treatment, Psychiatric comorbidities, Medication overuse, Disability
Key Summary Points
| Why carry out the study? | |
| Calcitonin gene-related peptide (CGRP) antagonists allow the extension of migraine prophylaxis to at least 12 months, unlike standard therapies, given their better efficacy-to-tolerability ratio. | |
| A randomized controlled trial (RCT) documented that long-term (1-year) fremanezumab treatment is effective, safe, and well tolerated in individuals with migraine and at most one prior therapeutic failure. | |
| Prospective real-life studies are needed to confirm the long-term efficacy, safety, and tolerability of fremanezumab in subjects with multiple therapeutic failures. | |
| What was learned from the study? | |
| Long-term fremanezumab treatment induced a rapid and sustained migraine improvement in people with migraine with > 3 preventive treatment failures, even in the presence of concomitant medication overuse or psychiatric comorbidities. The effectiveness and tolerability of fremanezumab in hard-to-treat individuals in real-life seem higher than those observed in RCTs. |
Introduction
Migraine is a progressive neurological disorder that may evolve into a chronic form if left untreated or improperly treated [1]. Adequate prophylactic treatment, recommended for patients with at least 3–4 disabling migraine days per month, should be extended over time, because migraine progression and chronicization are slowly evolving processes marked by substantial anatomical, physiological, and biochemical changes in the brain [2, 3]. Unfortunately, the limited tolerability of conventional migraine preventive drugs (beta-blockers, antiepileptics, tricyclics) has usually restricted migraine prophylaxis to only 4–6 months [4]. The possibility of providing extended migraine prevention is now feasible with the availability of drugs targeting the calcitonin gene-related peptide (CGRP), characterized by an excellent efficacy/tolerability ratio. Hence, the guidelines from the European Headache Federation recommend treating patients with migraine with anti-CGRP mAbs for at least 12–18 months before considering the possibility of discontinuation [5]. This recommendation is supported by prospective real-world evidence (RWE) studies, which indicate that despite their remarkable speed of action, the response to anti-CGRP mAbs may be late (≤ 6 months) or ultra-late (≤ 12 months) in a considerable proportion of subjects with migraine [6, 7].
In the prospective RWE FRIEND2 study, we documented that fremanezumab induced a ≥ 50% response rate (i.e., ≥ 50% reduction in monthly migraine/headache days compared to baseline) in 75.0% of patients with high-frequency episodic migraine (HFEM: ≥ 8 days/month) and in 72.9% of those with chronic migraine (CM) at 24 weeks. This was accompanied by a clinically meaningful reduction in monthly migraine days, monthly headache days, monthly analgesic intake, pain intensity, and migraine disability [8].
To assess whether the long-term treatment with fremanezumab results in a sustained efficacy/tolerability ratio also the in real world, we assessed its effectiveness, safety, and tolerability after 48 weeks of treatment in patients with HFEM or CM who had experienced multiple treatment failures and presented with numerous comorbidities.
Methods
This prospective, multicenter, cohort study is a part of the I-NEED project (Italian NEw migrainE Drugs database) and represents a subsidiary investigation within the framework of the Italian Migraine Registry (I-GRAINE).
We considered patients affected by high-frequency episodic migraine (HFEM) or chronic migraine (CM) who had not responded to at least three classes of preventive medications, including tricyclics, anticonvulsants, and beta-blockers (or onabotulinum toxin A for CM cases)—in accordance with the rules of the Italian Medicines Agency (AIFA, Agenzia Italiana del Farmaco) [9].
Patients were consecutively enrolled at 26 headache centers across 10 Italian regions (Latium, Calabria, Sicily, Campania, Liguria, Marches, Lombardy, Piedmont, Emilia-Romagna, Sardinia) starting from July 28, 2020. All patients were prescribed subcutaneously administered fremanezumab at a monthly dose of 225 mg or a quarterly dose of 675 mg, based on their preference, for a minimum of 48 weeks. We excluded subjects who had received onabotulinum toxin A within the past 12 weeks, those with previous exposure to anti-CGRP mAbs, or individuals with clinically significant cardiovascular conditions. No additional prophylactic medications were introduced during the study.
Comprehensive sociodemographic and clinical data were collected with face-to-face interviews conducted by specifically trained neurologists, using a standardized semi-structured questionnaire. Patients monitored migraine/headache days, recorded monthly analgesic medication use, assessed pain severity using the Numerical Rating Scale (NRS), evaluated migraine-related disability through the Headache Impact Test (HIT-6) and the Migraine Disability Assessment Scale (MIDAS), and reported any adverse events in a paper and pencil diary throughout the study duration.
The primary endpoint was the change in the number of monthly migraine days (MMD) for HFEM and of monthly headache days (MHD) for CM at weeks 45–48 compared to baseline. In patients with CM, we adopted the term headache day to denote any day on which a headache occurs, thereby including days characterized by migraine-like as well as tension-type headaches. This approach acknowledges the real-life behavior of patients with CM, who often initiate treatment at the onset of migraine pain, even when it is still categorized as a “headache,” thereby introducing a potential confounding factor in the attack classification. Secondary endpoints included the variation in (a) monthly analgesic medications, (b) NRS, and (c) HIT-6 at weeks 45–48 compared to baseline; (d) the change in MIDAS score at weeks 36–48 compared to baseline; (e) ≥ 50%, ≥ 75%, and 100% response rates at week 48 in all patients; (f) ≥ 50%, ≥ 75%, and 100% response rates at week 48 in patients with psychiatric comorbidities (anxiety, depression); (g) ≥ 50%, ≥ 75%, and 100% response rates at week 48 in patients with CM + medication overuse (MO); and (h) ≥ 50%, ≥ 75%, and 100% response rates at 48 weeks in patients with CM + MO + psychiatric comorbidities. Psychiatric comorbidities were identified through a specialist’s prior diagnosis, ongoing use of antidepressants (excluding amitriptyline), daily administration of anxiolytics, or a combination of these factors.
Written informed consent was obtained by all participants before their involvement in the study. The study was conducted in accordance with principles outlined in the Declaration of Helsinki. The study was approved by the IRCCS San Raffaele Roma Institutional Review Board (RP 19/26) and mutually recognized by the other local institutional review boards. The FRIEND3 study was not pre-registered on any clinical study registry.
Since some patients entered the study later and therefore were not treated for 48 weeks, a sensitivity analysis was performed to remove the possibility of selection bias. This analysis compared the baseline profiles of individuals treated for the entire 48 weeks with those treated for a shorter duration, ensuring that the two groups did not differ in terms of any prognostic parameters.
The characteristics of study participants were summarized using frequencies and percentages for categorical variables and means and standard deviations (SD) for continuous variables. Departure from normality was assessed using the Kolmogorov–Smirnov test. The chi-square test was employed for univariate analysis of categorical variables, with the Fisher’s exact test applied when the expected frequency fell below 5. Comparison of means between unpaired samples used the t test for independent samples, while the t test for paired samples was used when variables were compared before and after treatment. A P value < 0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS software package (SPSS Inc version 29.0).
Results
A total of 533 patients with migraine received at least one dose of fremanezumab and were considered for safety analysis (HFEM/CM 173/360; F/M 432/101; age 48.1 ± 11.8 years). Of these patients, 130 (HFEM/CM 49/81) were treated for at least 48 weeks (≥ 12 fremanezumab doses) and were considered for the effectiveness analysis (Fig. 1). The observed reduction in patient numbers from week 12 to 48 is not attributable to treatment discontinuation due to adverse events or lack of efficacy. Rather, it is simply because not all participants had been under observation long enough to be assessed at 48 weeks.
Fig. 1.
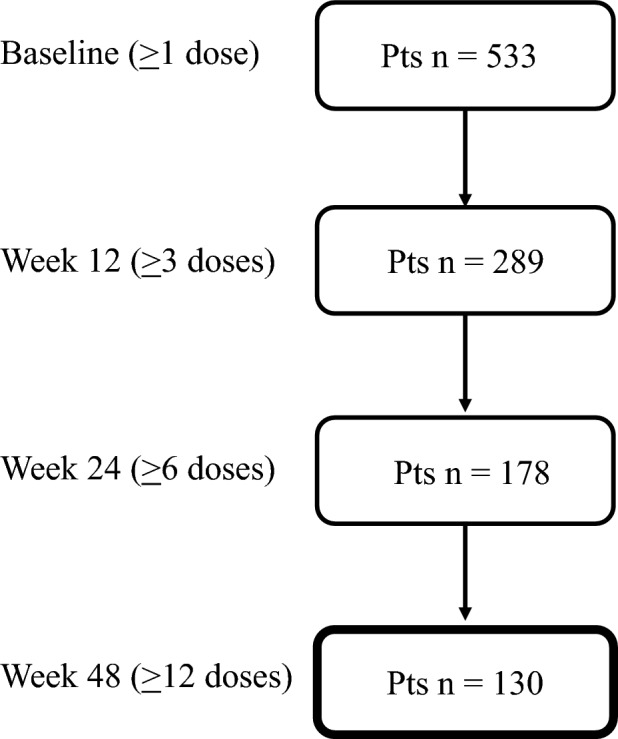
Patients’ disposition
The demographic and clinical features of the patients studied are described in Table 1. No data were missing. The sensitivity analysis, which compared the baseline profiles of individuals treated for the entire 48 weeks with those treated for a shorter duration, revealed no differences between the two groups regarding the severity of the disease, or any other parameters investigated. The only notable difference was observed in the MIDAS value, which was 19.7% higher in the groups of patients treated for the full 48-week period.
Table 1.
Demographic and clinical features of patients with high-frequency episodic migraine (HFEM) or chronic migraine (CM)
| Number (%) or mean ± SD | ||||
|---|---|---|---|---|
| All patients | HFEM | CM | P value | |
| Patients | 130 | 49 | 81 | – |
| Age, years | 49.5 ± 9.5 | 50.5 ± 9.7 | 48.9 ± 9.4 | 0.332 |
| Female | 99 (76.2) | 36 (73.5) | 63 (77.8) | 0.729 |
| BMI | 23.9 ± 3.6 | 24.2 ± 2.7 | 23.7 ± 4.2 | 0.517 |
| Age at onset, years | 17.2 ± 6.7 | 16.7 ± 6.1 | 17.4 ± 7.1 | 0.570 |
| MMD | 11.1 ± 1.8 | 11.1 ± 1.8 | – | – |
| MHD | 22.8 ± 5.1 | – | 22.8 ± 5.1 | – |
| NRS score | 8.1 ± 1.1 | 8.1 ± 0.9 | 8.1 ± 1.1 | 0.963 |
| MO | 62 (47.7) | – | 62 (76.5) | – |
| MO duration | 5.8 ± 8.7 | – | 5.8 ± 8.7 | – |
| Unilateral pain | 71 (54.6) | 27 (55.1) | 44 (54.3) | 0.931 |
| Unilateral cranial autonomic symptoms | 51 (39.2) | 21 (42.9) | 30 (37.0) | 0.636 |
| Allodynia | 74 (56.9) | 31 (63.3) | 43 (53.1) | 0.341 |
| Dopaminergic symptoms | 57 (43.8) | 19 (38.8) | 38 (46.9) | 0.469 |
| Monthly analgesic medications | 18.2 ± 8.6 | 11.7 ± 2.9 | 22.1 ± 8.5 | < 0.001 |
| Triptan responders | 96 (73.8) | 42 (85.7) | 54 (66.7) | 0.028 |
| Pts using concomitant prophylaxis | 46 (35.4) | 21 (42.8) | 25 (30.9) | 0.380 |
| Tricyclics | 18 (39.1) | 7 (33.3) | 11 (44.0) | |
| Anticonvulsants | 22 (47.8) | 10 (47.6) | 12 (48.0) | |
| Calcium channels antagonists | 4 (8.7) | 3 (14.3) | 1 (4.0) | |
| Beta-blockers | 16 (34.8) | 10 (47.6) | 6 (24.0) | |
| Vagal nerve stimulation | 1 (2.2) | 1 (4.8) | – | |
| Serotoninergic antagonists | 2 (4.3) | 2 (9.5) | – | |
| Prior treatment failures | 4.3 ± 1.3 | 3.9 ± 0.9 | 4.4 ± 1.5 | 0.031 |
| 3–4 | 89 (68.5) | 38 (77.6) | 51 (63.0) | 0.158 |
| > 4 | 41 (31.6) | 11 (22.5) | 30 (37.0) | |
| Onabotulinum toxin A responders* | 5 (27.8) | 4 (66.7) | 1 (8.3) | 0.041 |
| Pts with > 1 comorbidity | 68 (52.3) | 24 (48.9) | 44 (54.3) | 0.682 |
| Pts with psychiatric comorbidities | 43 (33.1) | 14 (28.6) | 29 (35.8) | 0.511 |
| HIT-6 score | 68.1 ± 4.0 | 67.2 ± 3.7 | 68.6 ± 4.1 | 0.058 |
| MIDAS score | 73.1 ± 55.4 | 57.5 ± 36.3 | 82.6 ± 62.6 | 0.012 |
| Fremanezumab dosing regimen | – | |||
| Monthly | 127 (97.7) | 49 (100) | 78 (96.3) | |
| Quarterly | 3 (2.3) | – | 3 (3.7) | |
HFEM high frequency episodic migraine, CM chronic migraine, BMI body mass index, MMD monthly migraine days, MHD monthly headache days, NRS numerical rating scale, MO medication overuse, HIT-6 Headache Impact Test-6, MIDAS Migraine Disability Assessment Scale, Pts patients, SD standard deviation
*Proportion calculated on the 85 subjects who were treated with onabotulinum toxin A
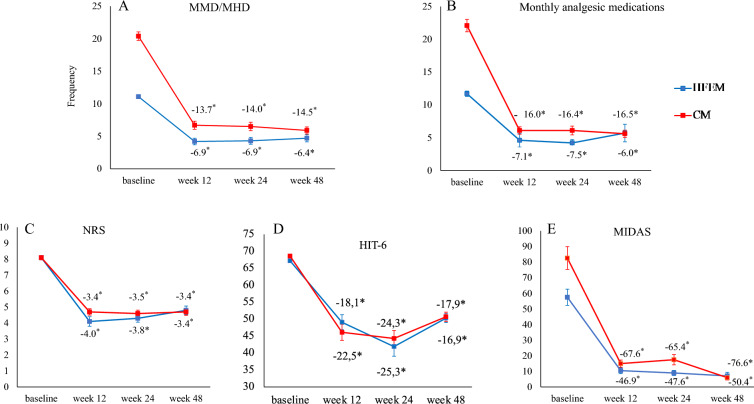
From baseline to weeks 45–48, fremanezumab significantly reduced MMD by − 6.4 ± 4.4 in HFEM (p < 0.001) and MHD by − 14.5 ± 6.9 in CM (p < 0.001) (Fig. 2, Supplementary Material Table 1). During the same time interval, fremanezumab decreased monthly analgesic medications by 6.0 ± 9.6, NRS by 3.4 ± 2.2, HIT-6 by 16.9 ± 9.2, and MIDAS by 50.4 ± 35.2 in patients with HFEM (p < 0.001). Similarly, for individuals with CM, fremanezumab resulted in a reduction of monthly analgesic medications by 16.5 ± 8.9, NRS by 3.4 ± 2.2, HIT-6 by 17.9 ± 11.9, and MIDAS by 76.6 ± 62.7 (p < 0.001) (Fig. 2, Supplementary Material Table 1).
Fig. 2.
Mean change in monthly migraine days/monthly headache days (MMD/MHD) (a), monthly analgesic medications (b), Numerical Rating Scale (NRS) score (c), Headache Impact Test-6 (HIT-6) score (d), and Migraine Disability Assessment Scale (MIDAS) score (e) from baseline to week 48 in patients affected by high-frequency episodic migraine (HFEM, n = 49) or chronic migraine (CM, n = 81)
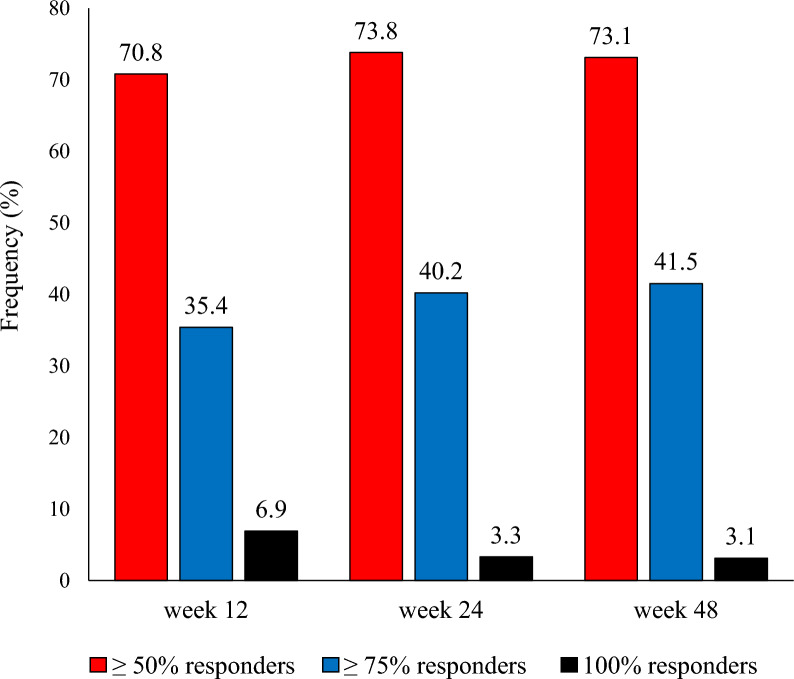
At week 48, the ≥ 50%, ≥ 75%, and 100% response rates to fremanezumab were as follows (Figs. 3, 4):
HFEM 75.5%, 36.7%, and 2%
CM 71.6%, 44.4%, and 3.7%
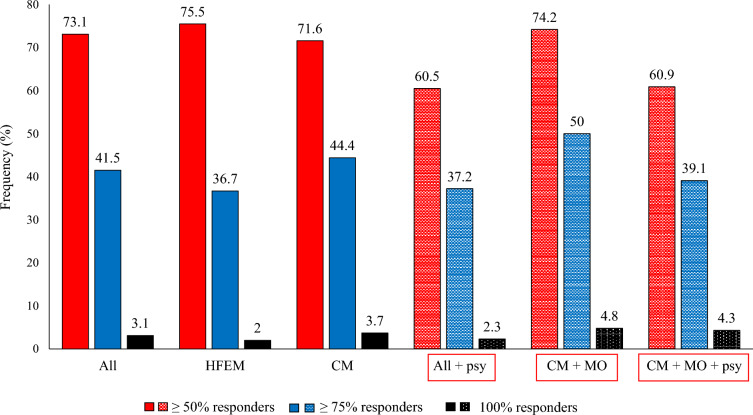
Patients with psychiatric comorbidities (43/130) 60.5%, 37.2%, and 2.3%
CM with MO (62/81) 74.2%, 50%, and 4.8%
CM with MO and psychiatric comorbidities (23/81) 60.9%, 39.1%, and 4.3%
Fig. 3.
Proportion of patients with a ≥ 50%, ≥ 75%, or 100% reduction in monthly migraine/headache days at week 12, 24, and 48 compared to baseline (data refer to all patients with migraine)
Fig. 4.
Proportion of patients with a ≥ 50%, ≥ 75%, or 100% reduction in monthly migraine/headache days at week 48 compared to baseline. All, all patients; HFEM, high-frequency episodic migraine; CM, chronic migraine; All + psy (dotted bars), all patients with migraine with psychiatric comorbidities; CM + MO (dotted bars), patients affected by CM with medication overuse; CM + MO + psy (dotted bars), patients affected by CM, medication overuse, and psychiatric comorbidities
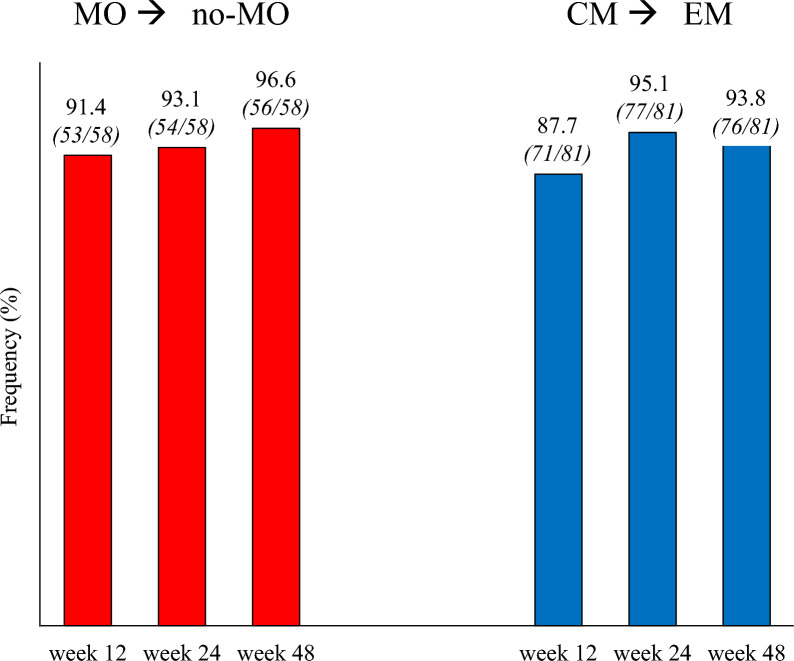
At week 48, the proportions of patients remitting from MO to no-MO and from CM to episodic migraine were 96.6% and 93.8%, respectively (Fig. 5).
Fig. 5.
Proportion of patients remitting from medication overuse (MO) to no medication overuse (no-MO) (red bars), and from chronic migraine (CM) to episodic migraine (EM) (blue bars)
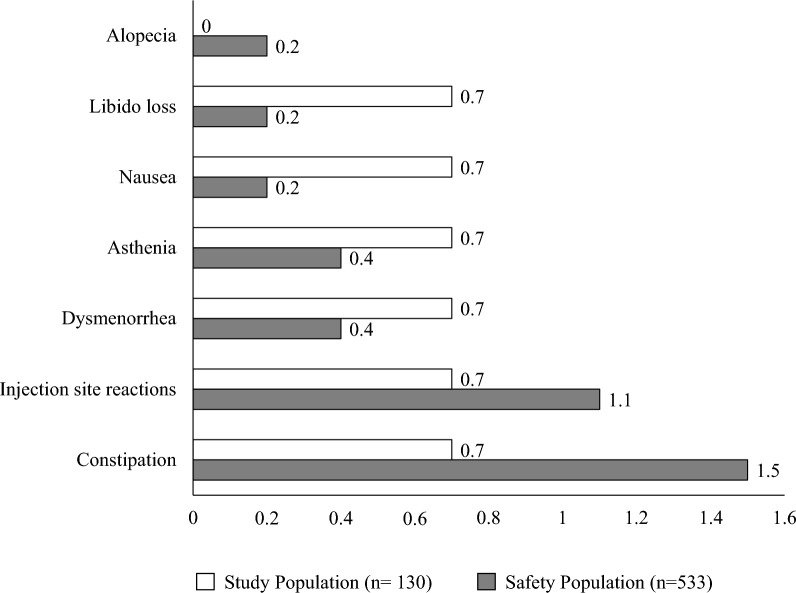
Treatment-emergent adverse events (TEAE) occurred in 3.9% (21/533) of patients in the safety population and in 7.8% (6/130) of patients treated with fremanezumab for at least 48 weeks (Fig. 6). All TEAE were rated as mild and transient, and no patient discontinued the treatment for any reason.
Fig. 6.
Treatment-emergent adverse events (TEAE) occurred in the study population [patients who had been treated for at least 48 weeks (≥ 12 fremanezumab doses), n = 130, white bars] and in the safety population [patients who had received ≥ 1 fremanezumab dose, n = 533, gray bars)]
Patients maintained an unchanged preventive regimen. This observation aligns with findings from other studies conducted by our group, involving erenumab and galcanezumab [10, 11]. The rationale behind this lies not only in the high proportion of > 50% responders but also in the significant reduction observed in pain intensity, acute medication use, and disability scores among patients with lower response rates.
Discussion
This prospective, multicenter, RWE study extends the findings of the 24-week FRIEND2 study documenting that fremanezumab treatment induces a significant reduction over 48 weeks in migraine frequency, analgesic intake, pain severity, and migraine disability in a population of patients with complex migraine affected by HFEM or CM with at least three treatment failures and frequent comorbidities (47.7%), predominantly psychiatric (33.1%). At week 48, the ≥ 50% response rate was achieved by three-fourths of the treated patients (73.1%), with 41.5% classified as super-responders (≥ 75% response rate) and 3.1% as absolute fremanezumab responders (100% response rate). Notably, a clinically meaningful response and super-response to fremanezumab was also observed in patients with psychiatric comorbidities, medication overuse, or both. Furthermore, almost all patients with medication overuse reverted to no-medication overuse (96.6%), and those with chronic migraine reverted to episodic migraine (93.8%) after one treatment year. The treatment was extremely well tolerated, with no safety issues emerging at any point.
Fremanezumab, a humanized monoclonal antibody (mAb) selectively targeting the CGRP pathway, has proven to be rapidly effective, safe, and tolerated in migraine prevention in randomized controlled trials (RCTs), regardless of prior therapeutic failures, medication overuse, and psychiatric comorbidities [12–14]. Its effects are sustained over time. In a multicenter RCT evaluating 1890 adults with chronic migraine (CM) or episodic migraine (EM), fremanezumab induced sustained improvements for up to 1 year in MMD (quarterly/monthly − 5.2/− 5.1), MHD (− 7.2/− 8.0), monthly days with any acute headache medication use (EM − 4.6/− 4.3; CM − 6/− 6.2), HIT-6 (CM − 7.8/− 8.4), and MIDAS (EM − 26.0/− 27.4) scores [15]. The study documented treatment-related adverse events in 54–59% of the patients, with very rare serious adverse events (5–7%) and treatment discontinuation due to adverse events (3–5%).
The effectiveness/tolerability ratio of fremanezumab at 1 year in the present RWE appears even better than that observed in the RCT. Specifically, we noted a more substantial reduction in MMD (− 6.4), MHD (− 14.5), HIT-6 scores (HFEM/CM − 16.9/− 17.9), and MIDAS scores (HFEM/CM − 50.4/− 76.6). Additionally, we identified higher proportions of ≥ 50% responders compared to those reported in the RCT (CM 71.4% vs. 53–57%; EM 75.5% vs. 66–68%). Finally, in the present study we found a much lower prevalence of TEAE compared to Goadsby’s study (7.8% vs. 54–59%), with no serious adverse event, nor discontinuation due to TEAE [15].
We already reported that the effectiveness of anti-CGRP mAbs in RWE is greater than the efficacy documented in RCTs [8, 16–20]. Our hypothesis is that patients in real life might exhibit a higher CGRP activity due to their increased migraine frequency and depressive comorbidities, conditions linked to elevated CGRP plasma or cerebrospinal fluid levels [19, 20]. This, in turn, would emphasize the therapeutic properties of anti-CGRP agents. Notably, we observed a much lower prevalence of adverse events compared to the RCT, and no patients discontinued the treatment for any reason, such as low tolerability or lack of efficacy.
A possible explanation for this discrepancy could be that our patients might have underestimated and underreported minor TEAE, considering their history of multiple prior therapeutic failures (on average 4.3) with traditional migraine preventive agents known for their low tolerability. Additionally, the inquiry and detection of TEAE in RWE studies could be less meticulous compared to an RCT setting.
The ability of fremanezumab to induce rapid and persistent reversal from MO to no-MO, as documented in the present long-term study, holds relevant clinical and economic implications, given the severe disability of these patients and their extremely high economic impact. The effectiveness of fremanezumab in patients with MO is not surprising. Its CGRP antagonism—a mechanism shared by gepants in acute migraine treatment—is likely to rapidly reduce their need for acute medication [21]. Furthermore, increased CGRP expression on trigeminal terminals in patients with MO could enhance the peripheral and central desensitizing effects of anti-CGRP mAbs [22].
While conventional prophylaxis often proves ineffective in patients with migraine affected by psychiatric comorbidities, our findings underscore the remarkable effectiveness of fremanezumab in this challenging-to-treat population, representing one-third (33.1%) of our study population. Notably, 60% of these patients with migraine achieved a ≥ 50% response and up to approximately 40% reached a ≥ 75% response rate. These results align with the outcome of the UNITE trial, an RCT demonstrating a clinically significant reduction in MMD/MHD following fremanezumab treatment in patients with migraine and comorbid depressive disorder [23]. The effectiveness of fremanezumab in these patients could—at least in part—be attributed to the putative involvement of CGRP in depression pathophysiology [20].
The present study is not without limitations. Firstly, our focus was exclusively on patients experiencing HFEM or CM who were referred to second- and third-level headache centers, thereby excluding individuals with lower migraine frequency. Additionally, participants utilized paper and pencil diaries instead of electronic diaries. Lastly, as a result of the open-label design of this long-term study, it is important to note that the observed improvement in migraine symptoms may, to some extent, be attributed to the regression to the mean phenomenon, as well as patient education and support throughout the study.
We also acknowledge the decision not to adjust our results for repeated measurements owing to the clear correlation between outcomes, as they all systematically evaluate the same predefined hypothesis. However, it’s important to note that even after applying a Bonferroni correction to account for multiple comparisons across all outcomes assessing the efficacy and safety of fremanezumab treatment, the significance of all tests was retained, with a p value < 0.01.
The main strengths of the study include its prospective multicenter design and the sizable and geographically representative population encompassing northern, central, and southern Italy. Notably, data collection was conducted by specifically trained headache specialists through face-to-face interviews, utilizing a shared web-based semi-structured questionnaire.
Conclusion
The long-term (1-year) preventive treatment with fremanezumab is highly effective and remarkably well tolerated in a real-life population of patients with HFEM or CM who have experienced multiple (> 3) therapeutic failures. The rapid and sustained improvements in migraine frequency, analgesic use, pain intensity, and migraine disability, along with the transitions from medication overuse (MO) to no-MO and from CM to episodic migraine, occur within the first month and persist over the 48-week treatment period. Importantly, these benefits are observed regardless of the presence of concomitant medication overuse or psychiatric comorbidities.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study and the Italian Migraine Registry study group: Maria Albanese, Marco Bertolini, Davide Bertuzzo, Maria Bloise, Francesco Bono, Licia Grazzi, Domenica Le Pera, Roberta Messina, Antonio Salerno, Silvia Strumia, Michele Trimboli, Luisa Fofi, Fabrizio Vernieri.
Editorial Assistance.
Editorial assistance was provided to the authors by Dr. Liliana Baiamonte.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
Piero Barbanti, Gabriella Egeo and Stefano Bonassi designed the study, Piero Barbanti and Gabriella Egeo drafted the manuscript, Stefano Bonassi and Stefania Proietti carried out data analysis, Gabriella Egeo, Cinzia Aurilia, Florindo d’Onofrio, Cinzia Finocchi, Laura Di Clemente, Maurizio Zucco, Alberto Doretti, Stefano Messina, Massimo Autunno, Angelo Ranieri, Antonio Carnevale, Bruno Colombo, Massimo Filippi, Miriam Tasillo, Steno Rinalduzzi, Pietro Querzani, Giuliano Sette, Lorenzo Forino, Francesco Zoroddu, Micaela Robotti, Alessandro Valenza, Cecilia Camarda, Laura Borrello, Marco Aguggia, Giovanna Viticchi, Carlo Tomino, Giulia Fiorentini, Bianca Orlando, Stefano Bonassi and Paola Torelli; Italian Migraine Registry study group performed data collection, Piero Barbanti, Gabriella Egeo and Stefano Bonassi revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was partially supported by the Italian Ministry of Health (Institutional Funding Ricerca Corrente) IRCCS San Raffaele and by Fondazione Italiana Cefalee (FICEF). IRCCS San Raffaele funded the journal’s Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of Interest
Piero Barbanti received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, ElectroCore, Eli-Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, Lusofarmaco, 1MED, MSD, New Penta, Noema Pharma, Novartis, Pfizer, Stx-Med, Teva, Visufarma, Zambon and serves as President with Italian Association of Headache Sufferers. Gabriella Egeo received travel grants and honoraria from Eli Lilly, Novartis, New Penta, Lundbeck and Ecupharma. Cinzia Aurilia received travel grants from FB-Health, Lusofarmaco, Almirall, Eli Lilly Novartis and Teva. Florindo d'Onofrio received travel grant, honoraria as a speaker or for partecipating in advisory boards from Novartis, Teva, Neopharmed Gentili, Qbgroupsrl, K link srl and Eli Lilly. Cinzia Finocchi received grants and honoraria from Novartis, Teva, Lilly, Lundbeck, Pfizer. Angelo Ranieri received speaker honoraria from TEVA, Ely Lilly, AIM Group. Antonio Carnevale has no disclosures to declare. Bruno Colombo has received congress fee reimbursements from Teva, Novartis, Pfizer. Massimo Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Alessandro Valenza received grants from Teva, Lilly, Lundbeck, Pfizer. Marco Aguggia received grants from Novartis and Lilly. Paola Torelli received travel grants and honoraria from Allergan, Teva, Eli Lilly and Novartis. Stefania Proietti, Laura Di Clemente, Maurizio Zucco, Alberto Doretti, Stefano Messina, Massimo Autunno, Miriam Tasillo, Steno Rinalduzzi, Pietro Querzani, Giuliano Sette, Lorenzo Forino, Francesco Zoroddu, Micaela Robotti, Cecilia Camarda, Laura Borrello, Giovanna Viticchi, Carlo Tomino, Giulia Fiorentini, Bianca Orlando and Stefano Bonassi have no disclosures to declare.
Ethical Approval
Eligible participants provided informed consent to participate in the study and were subsequently enrolled. The study was conducted in accordance with principles outlined in the Declaration of Helsinki. The study protocol received approval by IRCCS San Raffaele Roma Institutional Review Board (RP 19/26) and mutually recognized by other local institutional review boards.
Footnotes
The original online version of this article was revised to replace Fig. 2d.
Piero Barbanti and Gabriella Egeo made equal contributions to this work.
Change history
8/6/2024
A Correction to this paper has been published: 10.1007/s40120-024-00648-z
References
- 1.Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. 10.1186/s10194-019-1066-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rattanawong W, Rapoport A, Srikiatkhachorn A. Neurobiology of migraine progression. Neurobiol Pain. 2022;9(12):100094. 10.1016/j.ynpai.2022.100094. 10.1016/j.ynpai.2022.100094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 2021;397(10283):1505–18. 10.1016/S0140-6736(20)32342-4 [DOI] [PubMed] [Google Scholar]
- 4.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacco S, Amin FM, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J Headache Pain. 2022;23(1):67. 10.1186/s10194-022-01431-x. 10.1186/s10194-022-01431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbanti P, Aurilia C, Egeo G, et al. Late response to anti-CGRP monoclonal antibodies in migraine: a multicenter, prospective, observational study. Neurology. 2023;101(11):482–8. 10.1212/WNL.0000000000207292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbanti P, Aurilia C, Egeo G, et al. Ultra-late response (> 24 weeks) to anti-CGRP monoclonal antibodies in migraine: a multicenter, prospective, observational study. J Neurol. 2024. 10.1007/s00415-023-12103-4. 10.1007/s00415-023-12103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbanti P, Egeo G, Aurilia C, et al. Early and sustained efficacy of fremanezumab over 24-weeks in migraine patients with multiple preventive treatment failures: the multicenter, prospective, real-life FRIEND2 study. J Headache Pain. 2023;24(1):30. 10.1186/s10194-023-01561-w. 10.1186/s10194-023-01561-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzetta Ufficiale della Repubblica Italiana. Serie Generale n; 2020. https://gazzettaufficiale.it/gazzetta/serie_generale/caricaDettaglio?dataPubblicazioneGazzetta=2020%E2%80%9307%E2%80%9321&numeroGazzetta=182. Accessed Jan 17, 2023.
- 10.Barbanti P, Aurilia C, Cevoli S, et al. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: results of the EARLY 2 study. Headache. 2021;61(9):1351–63. 10.1111/head.14194 [DOI] [PubMed] [Google Scholar]
- 11.Vernieri F, Brunelli N, Marcosano M, et al. Maintenance of response and predictive factors of 1-year galcanezumab treatment in real-life migraine patients in Italy: the multicenter prospective cohort GARLIT study. Eur J Neurol. 2023;30(1):224–34. 10.1111/ene.15563. 10.1111/ene.15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008. 10.1001/jama.2018.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–22. 10.1056/NEJMoa1709038 [DOI] [PubMed] [Google Scholar]
- 14.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–40. 10.1016/S0140-6736(19)31946-4 [DOI] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Silberstein SD, Yeung PP, et al. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: a randomized study. Neurology. 2020;95(18):e2487–99. 10.1212/WNL.0000000000010600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbanti P, Egeo G, Aurilia C, et al. Fremanezumab in the prevention of high-frequency episodic and chronic migraine: a 12-week, multicenter, real-life, cohort study (the FRIEND study). J Headache Pain. 2022;23(1):46. 10.1186/s10194-022-01396-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbanti P, Aurilia C, Egeo G, et al. Erenumab in the prevention of high-frequency episodic and chronic migraine: erenumab in real life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache. 2021;61(2):363–72. 10.1111/head.14032 [DOI] [PubMed] [Google Scholar]
- 18.Vernieri F, Altamura C, Brunelli N, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study). J Headache Pain. 2021;22(1):35. 10.1186/s10194-021-01247-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cernuda-Morollón E, Larrosa D, Ramón C, et al. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191–6. 10.1212/WNL.0b013e3182a6cb72 [DOI] [PubMed] [Google Scholar]
- 20.Mathe AA, Agren H, Lindstrom L, Theodorsson E. Increased concentration of calcitonin gene-related peptide in cerebrospinal fluid of depressed patients. A possible trait marker of major depressive disorder. Neurosci Lett. 1994;182(2):138–142. [DOI] [PubMed]
- 21.Rissardo JP, Caprara ALF. Gepants for acute and preventive migraine treatment: a narrative review. Brain Sci. 2022;12(12):1612. 10.3390/brainsci12121612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saengjaroentham C, Strother LC, Dripps I, et al. Differential medication overuse risk of novel antimigraine therapeutics. Brain. 2020;143:2681–8. 10.1093/brain/awaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton R, Ramirez Campos V, Roth-Ben Arie Z, et al. Efficacy of fremanezumab treatment in reducing monthly migraine days in patients with migraine and major depressive disorder: results from the UNITE study. In: American Headache Society 65th Annual Scientific Meeting June 15–18, 2023 Austin, TX, United States P-231.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.