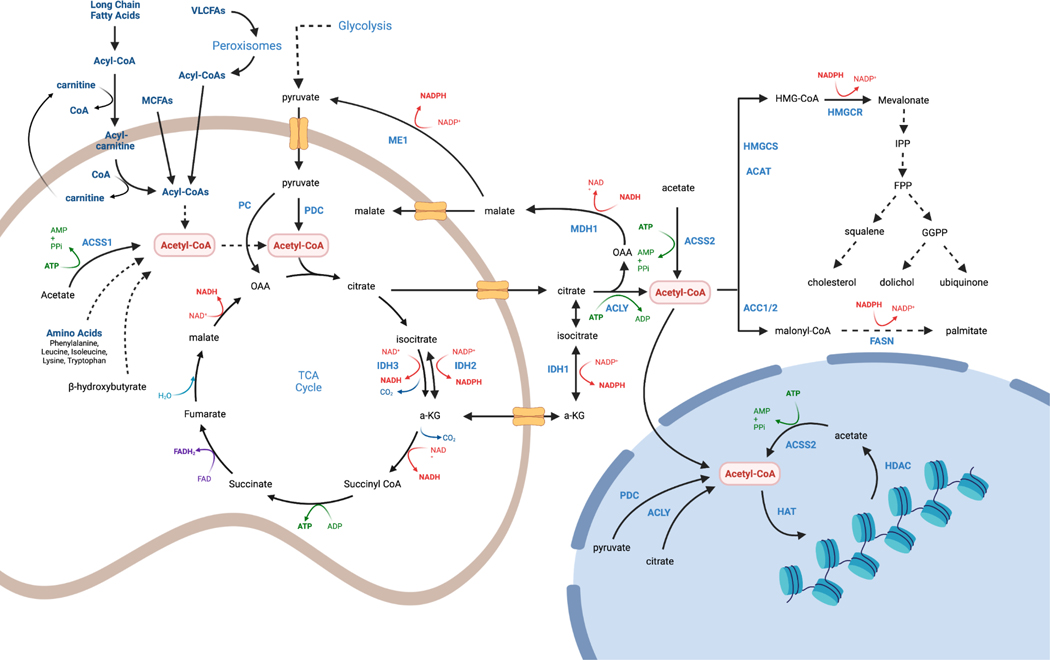
Figure 1. Compartmentalized acetyl-CoA production pathways.
Mitochondrial acetyl-CoA is generated in many cell types from glucose during anabolic conditions, and from other nutrients, such as long and medium chain fatty acids, acetate, amino acids and beta-hydroxybutyrate, during catabolic conditions, though different cell and tissue types have different nutrient preferences. Pyruvate enters mitochondria through the mitochondrial pyruvate carrier (MPC) and undergoes oxidative decarboxylation by pyruvate dehydrogenase complex (PDC) yielding acetyl-CoA, NADH and CO2. Fatty acids are transported into mitochondria as acyl-carnitines via the Cpt1/Cpt2 shuttling system, which operates between the mitochondrial outer and inner membranes (not shown). Peroxisomes can also generate short chain fatty acids that are delivered to mitochondria; medium chain fatty acids do not require the acyl-carnitine shuttle. Acetyl group carbons enter the TCA cycle following a condensation reaction with oxaloacetate catalyzed by citrate synthase (CS). TCA cycle flux generates CO2 and the reducing equivalents that drive the electron transport chain (ETC) and ATP synthesis. Cytosolic acetyl-CoA is used for fatty acid synthesis and in the mevalonate pathway. Cytosolic acetyl-CoA carbons are transferred from mitochondrial citrate via the mitochondrial citrate carrier (Slc25a1). Citrate is cleaved by ATP Citrate Lyase (ACLY) to make cytosolic acetyl-CoA. Citrate may also be generated through reductive carboxylation by the isocitrate dehydrogenases (IDH1/IDH2). Alternatively, cytosolic acetyl-CoA can be derived from acetate via acyl-CoA short-chain synthetase-2 (ACSS2). Acetyl-CoA generated in the cytosol may diffuse into the nucleus for histone acetylation reactions. However, ACLY, ACSS2, and PDC have all been reported in the nucleus where they may generate local acetyl-CoA.