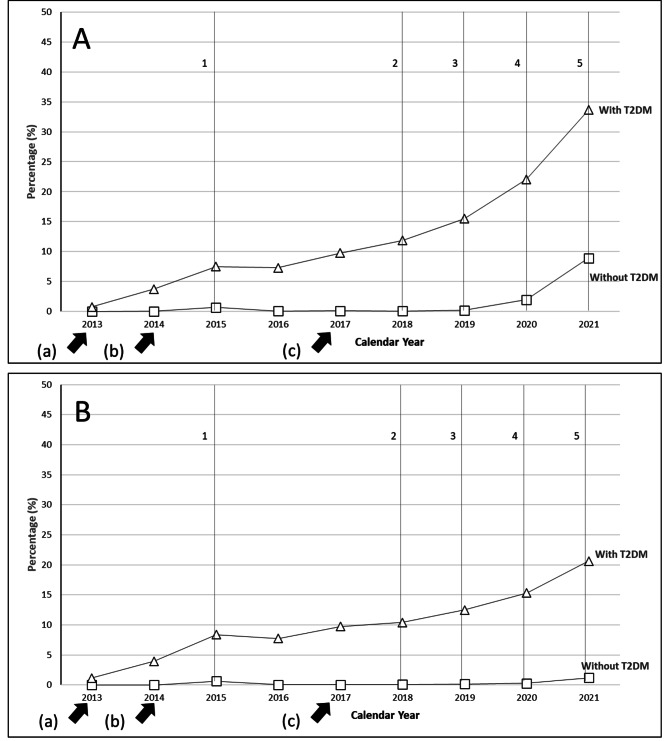
Fig. 2.
Trends in prescribing patterns of SGLT2i medications among patients with HFrEF and HFpEF stratified by T2DM. Relative prevalence of all SGLT2i prescribing (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin) within the HFrEF cohort (A) and HFpEF cohort (B) identified in MarketScan commercial and Medicare claims between January 2013 and December 2021. a) FDA approval of canagliflozin, b) FDA approval of both dapagliflozin in Q1 and empagliflozin in Q3, c) FDA approval of ertugliflozin. 1: EMPA-REG is published demonstrating CVD benefits in patients with T2DM. 2: American Diabetes Association guidelines recommends SGLT2i as a second-line therapy for patients with T2DM and CVD. 3: DAPA-HF demonstrates dapagliflozin reduces risk of worsening HF and CV mortality regardless of T2DM status. 4: Dapagliflozin receives FDA indication for HF. 5: EMPEROR-PRESERVED finds CV benefits with empagliflozin in HFpEF patients; update to ACC heart failure decision algorithm promotes dapagliflozin and empagliflozin as a first-line component of guideline-directed medical therapy