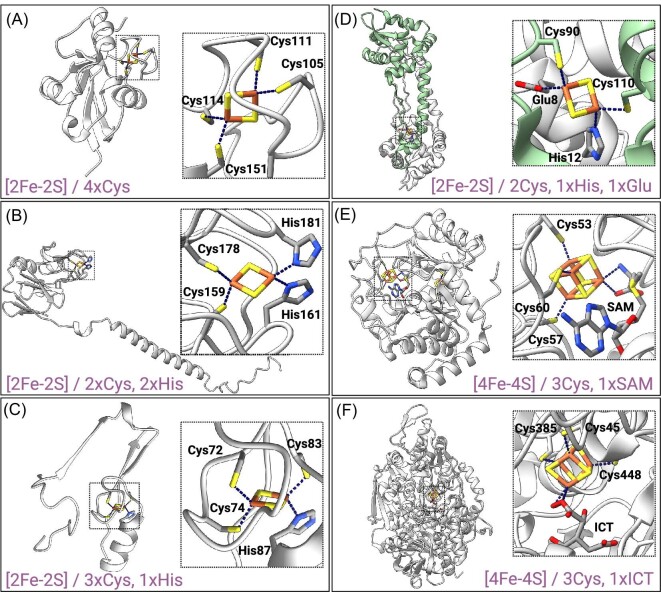
Fig. 1.
Examples of Fe-S proteins with different Fe-S cluster structure and coordination. (A) Human ferredoxin 2 (PDB ID: 2Y5C11); [2Fe-2S] coordinated with four Cys residues. (B) Rieske subunit of Saccharomyces cerevisiaebc1 complex (PDB ID: 1KYO13); [2Fe-2S] coordinated with two Cys residues and two His residues. (C) Human mitoNEET (only one subunit of the homodimer is represented) (PDB ID: 7P0O15); [2Fe-2S] coordinated with three Cys residues and one His residue. (D) Transcription regulator RsrR from Escherichia coli (PDB ID: 6HSD17); [2Fe-2S] coordinated with two Cys residues from one subunit of the homodimer (in green) and by His and Glu residues from the other. (E) Biotin synthase from E. coli (PDB ID: 1R3019); [4Fe-4S] cluster coordinated with three Cys residues and an exchangeable S-adenosyl-L-methionine (SAM) molecule (on the right), and [2Fe-2S] coordinated with three Cys and one Arg residues (Fig. 1). (F) Aconitase from Bos taurus (PDB ID: 1C9721); [4Fe-4S] cluster coordinated with three Cys residues and a molecule of isocitrate (ICT).