Fig. 2.
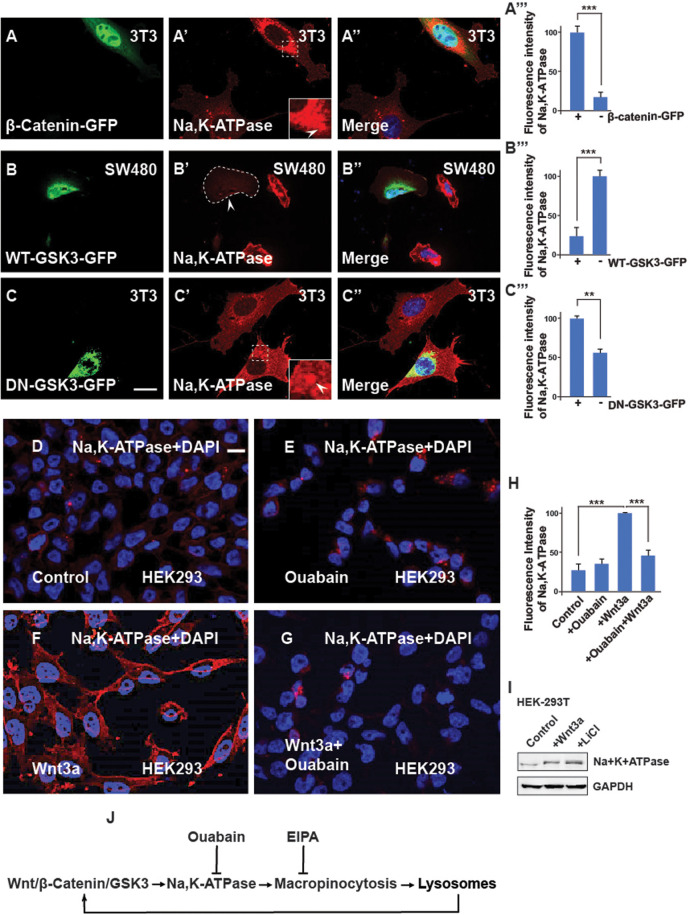
The Na,K-ATPase is required for Wnt signaling and its regulation is via GSK3. (A-A‴) 3T3 cells transfected with the stabilized constitutively active forms of β-catenin-GFP show increased levels of Na,K-ATPase compared to untransfected neighbor cells used as a control. Quantification is shown on the right side. (B-B‴) Overexpression of WT-GSK3-GFP (demarcated by a stippled line) in CRC SW480 cells blocks the Na,K-ATPase stabilization by Wnt. (C-C‴) Transfecting DN-GSK3-GFP (DN-GSK3-GFP) in 3T3 cells increases Na,K-ATPase levels. Note that the accumulation is observed in vesicles (indicated by the stippled box). (D) HEK293BR (BAR/Renilla) cells stained with Na,K-ATPase antibody. (E) HEK293BR cells treated with the Na,K-ATPase inhibitor Ouabain (1 µM) overnight showed no changes in Na,K-ATPase levels. (F) Overnight treatment with Wnt3a protein (100 ng/ml from Peprotech) treatment stabilized Na,K-ATPase. (G) Ouabain blocked Na,K-ATPase stabilization due to Wnt3a. (H) Quantification of the fluorescence intensity of the Na,K-ATPase from D-G. (I) Western blot showing that endogenous levels of Na,K-ATPase increase after activating the canonical Wnt pathway. (J) Diagram showing that Na,K-ATPase is positively regulated by Wnt via macropinocytosis in lysosomes. EIPA blocks macropinocytosis; Ouabain blocks the Na,K-ATPase, and lysosomes enhance Wnt. All experiments with cultured cells were biological triplicates. Scale bars: 10 μm. Error bars denote s.e.m. (n≥3) (**P<0.01, ***P<0.001).