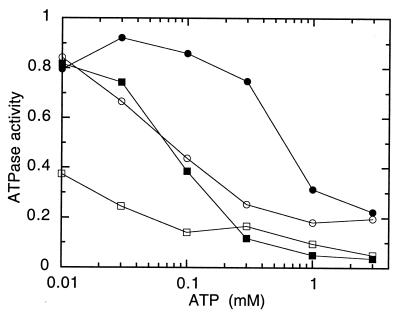
FIG. 2.
Dependence of vanadate inhibition on ATP concentration. Proteoliposomes containing MalF500GK2 were preincubated for 20 min at 23°C in the presence of vanadate and nucleotide, diluted 60-fold, and collected by centrifugation. Preincubations contained 3 mM MgCl2, ATP, and, in addition, 1.5 μM MalFGK2 and 0.1 mM vanadate (●); 1.5 μM MalFGK2 and 0.5 mM vanadate (○); 0.15 μM MalFGK2 and 0.1 mM vanadate; (■) or 0.15 μM MalFGK2 and 0.5 mM vanadate (□). ATPase activity is presented as a fraction of the rate seen in the absence of vanadate (3.2 μmol/min/mg of protein). Each point is the mean of three separate determinations that varied within 0.05 of the mean.