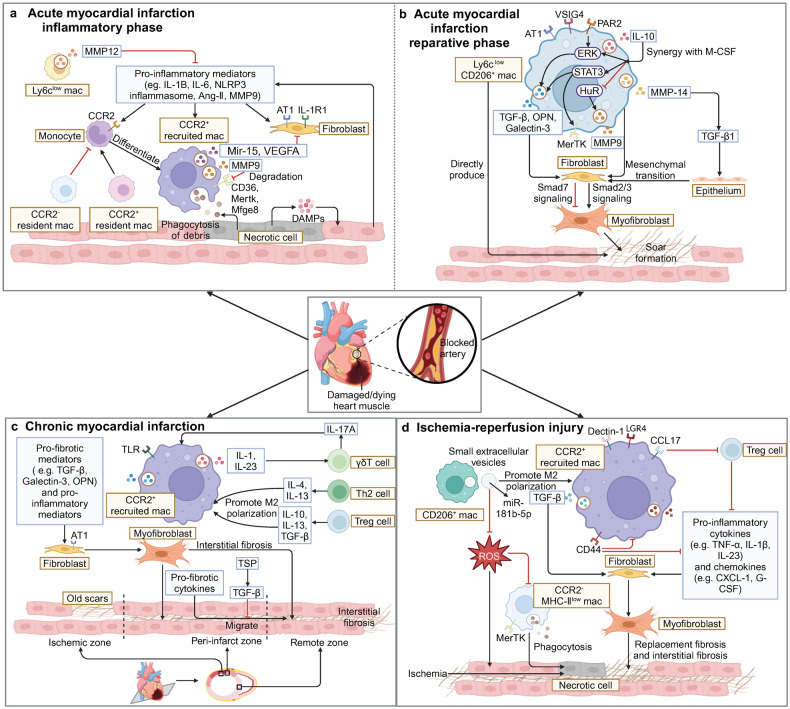
Fig. 2.
Regulations of myocardial fibrosis by macrophages after ischemic injury. a In the inflammatory phase of AMI, DAMP activates retained cells in the heart to release pro-inflammatory mediators, thereby promoting monocyte infiltration and differentiation into CCR2+ macrophages. CCR2+ macrophages secrete mediators (such as MMPs, miR-15, and VEGFA) to regulate inflammation and fibrosis in order to clear necrotic tissue and prepare for subsequent cardiac repair. b In the reparative phase of AMI, restorative Ly6ClowCD206+ macrophages become the main macrophage subset in the heart. They secrete anti-inflammatory and pro-fibrotic mediators such as TGF-β, IL-10, galectin-3, and IL-10 to promote the conversion of fibroblasts into myofibroblasts which secrete collagen to form scars. c When CMI occurs, CCR2+ macrophages continue to infiltrate into the heart, interact with T cells, and secrete a large amount of pro-inflammatory and pro-fibrotic factors, causing interstitial fibrosis in remote zone. d When IRI occurs in the heart, a large number of CCR2+ macrophages accumulate in the early stage. They upregulate LGR4, Dectin-1 and CCL17 to promote inflammation and myocardial fibrosis, or upregulate the expression of CD44 and receive small extracellular vesicles secreted by M2 macrophages to convert to a reparative phenotype and attenuate the inflammatory response caused by ROS. (Created with BioRender.com)