Abstract
Objective.
Because zygapophyseal joints (ZJ) are difficult to visualize on radiographs, little is known about the relationship of ZJ fusion to other features of spinal damage in ankylosing spondylitis (AS). We used computed tomography (CT) to investigate the concordance of ZJ fusion and syndesmophytes, and examined the contribution of both features to spinal motion.
Methods.
We performed thoracolumbar CT scans (T10–T11 to L3–L4) on 55 patients. Two readers scored scans for ZJ fusion, which were compared to syndesmophyte height and extent of bridging, measured by computer algorithm at the same levels. We used multiple regression analysis to evaluate the relative contributions of ZJ fusion and syndesmophytes to spinal mobility.
Results.
Fifty-one percent of patients had ZJ fusion in at least 1 vertebral level. Fusion was present in 129 of 652 individual ZJ. Syndesmophytes and bridging were often present in vertebral levels without ZJ fusion, suggesting that syndesmophytes most often develop first. ZJ fusion was present in 34% of vertebral levels with syndesmophytes and 55.9% of levels with bridging, suggesting a closer association with bridging. Syndesmophytes and ZJ fusion had similar associations with the modified Schober test, but syndesmophytes were more strongly associated with limitations in lateral thoracolumbar flexion. ZJ rarely showed new fusion over 4 years.
Conclusion.
Thoracolumbar ZJ fusion in AS is rarely present at vertebral levels without syndesmophytes. Syndesmophytes, therefore, likely appear before ZJ fusion at a given vertebral level. Both syndesmophytes and ZJ fusion contribute to limited forward lumbar flexion, but syndesmophytes contribute more to limited lateral flexion.
Keywords: ANKYLOSING SPONDYLITIS, SYNDESMOPHYTE, ZYGAPOPHYSEAL JOINT, COMPUTED TOMOGRAPHY
Spinal fusion in ankylosing spondylitis (AS) can affect both the posterior and anterior aspects of vertebrae. Ossification of the zygapophyseal (facet) joints (ZJ), which are commonly inflamed in AS, fuses the posterior elements1,2. Similarly, ossification at the annulus fibrosis in the periphery of the intervertebral disc in the form of syndesmophytes can lead to fusion of the vertebral bodies. The small size and curved configuration of ZJ, and the overlapping shadows from other vertebral structures, makes it difficult to visualize abnormalities of ZJ on radiographs, particularly in the lumbar spine3,4,5. Consequently, studies of spinal structural damage in AS have focused on syndesmophyte growth because syndesmophytes are easier to visualize than ZJ.
Little is known about the process of ZJ fusion in AS, including whether ZJ fusion typically antedates or follows syndesmophyte formation and bridging. If ZJ fusion occurs before, or more frequently than, syndesmophyte formation, the ZJ may be a more informative site to monitor in studies of the disease-modifying potential of medications. The relative contribution of ZJ fusion and syndesmophytes to spinal rigidity in AS also remains an open question. Two small studies reported correlations between abnormalities in lower lumbar ZJ and limited lumbar motion, but did not simultaneously examine syndesmophytes6,7. If limited lumbar motion in AS is primarily due to ZJ fusion, investigation of ways to prevent ZJ fusion would assume added significance.
Previous studies have mainly used plain radiographs to evaluate ZJ fusion8. Radiography, which collapses a 3-D structure into a 2-dimensional image, offers only limited visualization of syndesmophytes and even poorer visualization of ZJ. Computed tomography (CT) can provide a clear 3-D view of ZJ and syndesmophytes. We have previously used CT to quantitate syndesmophytes in the thoracolumbar spine of patients with AS9,10,11. The scans encompass the whole vertebra including the posterior elements and ZJ, providing a unique opportunity to elucidate the concordance between ZJ fusion and syndesmophytes and vertebral bridging in AS, and the relative contribution of each abnormality to restricted lumbar motion. The goal of the study was to determine whether ZJ fusion should be preferred over syndesmophytes as a measure of spinal fusion in AS.
MATERIALS AND METHODS
Patients.
Patients were enrolled at the US National Institutes of Health and Johns Hopkins Medical Institutions. The study protocol was approved by the National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board (04-AR-0205) and the Johns Hopkins Institutional Review Board (CR00009082), and patients provided written informed consent. Inclusion criteria were age 18 or older, classification of AS by the modified New York criteria12, and a Bath AS Radiology Index (BASRI) lumbar score of 0, 1, 2, or 313. We excluded patients with completely fused lumbar spines. Lumbar mobility was evaluated using the modified Schober test and lateral thoracolumbar flexion. Mobility measurements were performed by 1 examiner (MMW). We used the Bath AS Disease Activity Index (BASDAI) to measure symptoms14.
CT scanning.
Four scanners were used during the study: a Philips Brilliance 64 (slice thickness 1.5 mm), a GE Lightspeed Ultra (slice thickness 1.25 mm), a Siemens Somatom Flash (slice thickness 1.0 mm), and a Siemens Somatom Force (slice thickness 1.0 mm). Voltage and current variables were 120 kVp and 300 mAs, respectively, for the first 2 scanners, and 120 kVp and 190 mAs for the last 2 scanners. The scans included 6 vertebral levels that were processed: T10–T11, T11–T12, T12–L1, L1–L2, L2–L3, and L3–L4. The estimated equivalent absorbed radiation dose was 8.01 mSv.
CT quantitative image analysis of syndesmophytes.
We evaluated the circumferential height of syndesmophytes along the vertebral body rim using a previously validated method9. Syndesmophyte height was measured in 72 angular sectors of 5° each around the rim10. In each angular sector, we recorded the height of the tallest syndesmophyte and normalized it to the intervertebral disc height (a score of 0 indicating no syndesmophyte and score of 1 indicating bridging). Scores of the 72 angular sectors were summed to form the circumferential height for each intervertebral disc space (a score of 72 indicating complete fusion). We also computed the extent of bridging in an intervertebral disc space as the sum of angular sectors with a score of 1. For this measure, a value of 360° represented complete fusion.
ZJ fusion on CT.
Two readers, a musculoskeletal radiologist (LY) and a rheumatologist (MMW), visually assessed the ZJ on the CT scans. Before readings were done, the scans were anonymized and vertebral bodies were digitally masked to blind readers to the presence or absence of syndesmophytes. At each vertebral level, readers scored each ZJ after examining it in axial and sagittal planes. ZJ were initially scored as normal, abnormal but not fused (erosive changes, arthrosis, or mixed), or fused, but interreader agreement for this 3-category scale was only moderate (κ 0.56). Therefore, for analysis we considered only the dichotomous reading of fused or not fused. A joint was scored as fused if cortical bridging affected part or the entire joint space. We did not consider capsular calcification to represent joint fusion.
Longitudinal study.
A subset of patients had repeat CT scans 2 years and 4 years after the first scan. Scans were masked as in the baseline study, and were read for ZJ fusion by 1 reader (MMW). Differences from baseline in the status of ZJ fusion and circumferential syndesmophyte height were recorded.
Statistical analysis.
Interreader reliability of ZJ scores was assessed using κ statistics based on readings of individual ZJ. For analyses of associations with syndesmophytes and bridging, which have the vertebral level as the unit of analysis, we considered ZJ fusion to be present if either ZJ at a given level was fused. We tested for trends in the frequency of ZJ fusion by vertebral level (from T10–T11 to L3–L4) using the nonparametric Jonckheere-Terpstra test. With the patient as the unit of analysis, we used Spearman correlations to evaluate the association between the number of thoracolumbar vertebral levels with any ZJ fusion (possible range 0–6) and lumbar motion measurements.
We used multiple regression analysis to evaluate the relative contribution of ZJ fusion and syndesmophytes (or bridging) to the modified Schober test and lateral thoracolumbar flexion. For lateral thoracolumbar flexion, we used the more limited of right and left movements as the dependent variable in the analyses, because this would be a more specific indicator if ZJ fusion was primarily unilateral. In these analyses, we used either the sum of syndesmophyte heights or the sum of the extent of bridging across all vertebral levels as the per-patient measures of syndesmophyte involvement. The BASDAI was included as an independent variable in the models to adjust for differences in AS activity. We did not include the C-reactive protein level because this measure was not correlated with either the modified Schober test (r = −0.07, p = 0.62) or lateral thoracolumbar flexion (r = 0.16, p = 0.27) and was missing for 7 patients. Associations from the multiple regression models were represented as standardized β coefficients, which allow direct comparisons of the strength of association of ZJ fusion and syndesmophyte measures with lumbar motion measurements. This is particularly important when the scales of the independent variables differ. We used SAS software (version 9.3) for data analysis.
RESULTS
Patients.
We studied 55 patients, including 47 men (85%), with mean (SD) age of 45.1 years (11.6), mean duration of AS of 18.5 years (11.1), and mean BASDAI of 2.8 (1.9). Their mean modified Schober test was 3.5 cm (1.2; range 0.5–6.4 cm), and their mean lateral thoracolumbar flexion (worst of right and left measurements) was 13.1 cm (5.1; range 3.0–25.0 cm). A total of 326 vertebral levels (of 330 possible levels) were available for analysis at baseline. Four patients were scanned too low, providing no data for the T10–T11 level.
ZJ fusion at baseline.
Figure 1 shows examples of fused ZJ. Agreement on the presence of ZJ fusion between the 2 physicians’ readings was very good (κ 0.84, 95% CI 0.79–0.89). Of 652 individual ZJ, both readers agreed on the status of 619 joints (114 fused and 505 not fused). Overall, 129 joints (19.8%) were read as fused by 1 reader and 132 joints (20.2%) were read as fused by the second reader.
Figure 1.
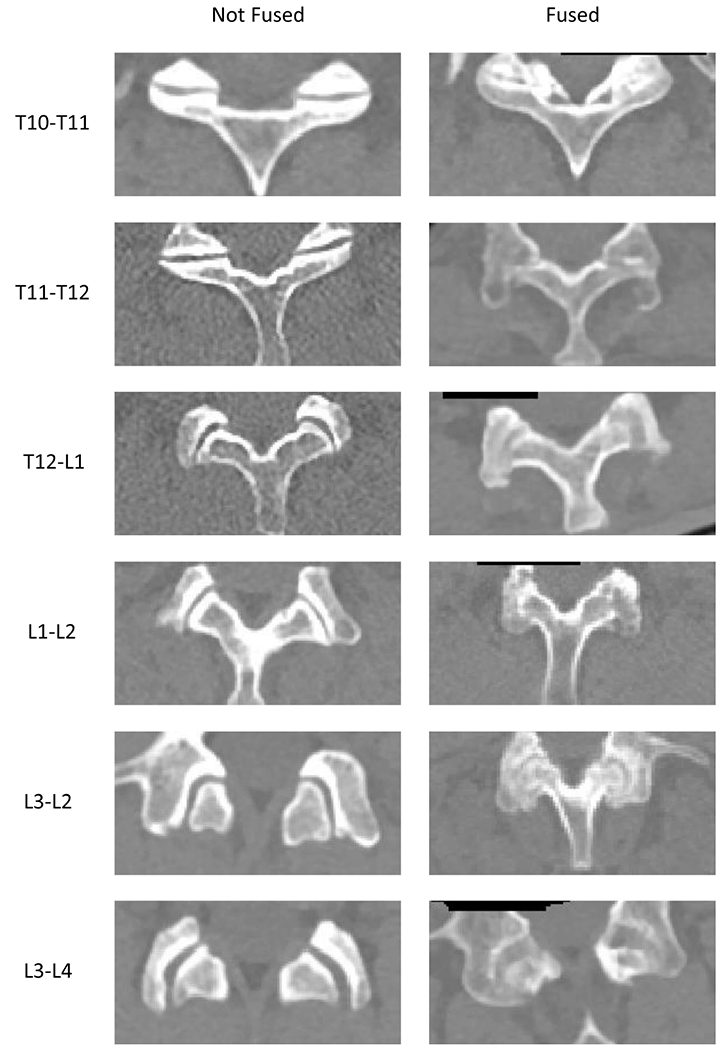
Examples of fused and non-fused zygapophyseal joints from several patients viewed using computed tomography.
As read by both readers, 51% of patients had ZJ fusion at 1 or more vertebral levels and 49% had no ZJ fusion. The median number of vertebral levels with ZJ fusion was 1. The median number of fused ZJ among patients with any fused ZJ was 4. Few patients (14.6%) had ZJ fusion in more than 3 vertebral levels, and only 2 patients (3.6%) had ZJ fusion at all 6 levels. In levels with ZJ fusion, the fusion was bilateral in 62%/67% (reader 1/reader 2).
ZJ fusion was more frequent in the more superior vertebral levels, and the frequency was much lower below the T12–L1 level (Table 1). Most levels with ZJ fusion were contiguous. If 1 vertebral level had ZJ fusion, the immediately superior level also had ZJ fusion in 72%/74% (reader 1/reader 2) of patients.
Table 1.
Percentage of patients with fusion in ZJ, syndesmophytes, or bridging by vertebral level (n = 55).
| Vertebral | ZJ Fusion | Syndesmophytes | Bridging | |
|---|---|---|---|---|
| Level | Reader 1 | Reader 2 | ||
| T10–T11* | 33.3 | 35.5 | 74.5 | 56.9 |
| T11–T12 | 38.2 | 36.3 | 72.7 | 47.3 |
| T12–L1 | 34.5 | 34.5 | 67.3 | 41.8 |
| L1–L2 | 12.7 | 16.4 | 63.6 | 16.4 |
| L2–L3 | 14.6 | 12.7 | 63.6 | 18.2 |
| L3–L4 | 7.3 | 10.9 | 56.4 | 9.1 |
| ptrend | < 0.0001 | < 0.0001 | 0.03 | < 0.0001 |
Data available for 51 patients. ZJ: zygapophyseal joint.
The number of individual fused ZJ correlated with patient age (r = 0.35, p = 0.009) and duration of AS (r = 0.44, p = 0.0008).
Association between ZJ fusion and syndesmophytes.
We examined the association between ZJ fusion in either joint and the presence of syndesmophytes in the same vertebral level. Syndesmophytes were present in 216 of 326 levels (66%), and bridging was present in 102 levels (31%). The most common configuration [45%/44% of 326 levels (reader 1/reader 2)] was a vertebral level with syndesmophytes but no ZJ fusion (Table 2). Conversely, in only 6 levels (1.8%) was there ZJ fusion and no syndesmophytes. Of 216 levels with syndesmophytes, only 32%/34% (reader 1/reader 2) also had ZJ fusion. These results indicate that syndesmophytes are often present in vertebral levels that do not have ZJ fusion and rarely the converse, suggesting that ZJ fusion most often follows the development of syndesmophytes.
Table 2.
Association between ZJ fusion and syndesmophytes, and between ZJ fusion and bridging syndesmophytes in the same vertebral level (n = 326).
| Variable | Configuration | Reader 1 | Reader 2 |
|---|---|---|---|
| Syndesmophyte | ZJ fusion−, syndesmophyte− | 104 (32%) | 104 (32%) |
| ZJ fusion−, syndesmophyte+ | 146 (45%) | 143 (44%) | |
| ZJ fusion+, syndesmophyte− | 6 (2%) | 6 (2%) | |
| ZJ fusion+, syndesmophyte+ | 70 (21%) | 73 (22%) | |
| Bridging | ZJ fusion−, bridging− | 207 (64%) | 202 (62%) |
| ZJ fusion−, bridging+ | 43 (13%) | 45 (14%) | |
| ZJ fusion+, bridging− | 17 (5%) | 22 (7%) | |
| ZJ fusion+, bridging+ | 59 (18%) | 57 (17%) |
Numbers indicate no. vertebral levels verifying each condition: −: absent; +: present. ZJ: zygapophyseal joint.
The proportion of vertebral levels with any ZJ fusion increased progressively with the extent of syndesmophyte involvement (Figure 2A). All levels with syndesmophyte circumferential height of 50 or more had fusion of at least 1 ZJ.
Figure 2.
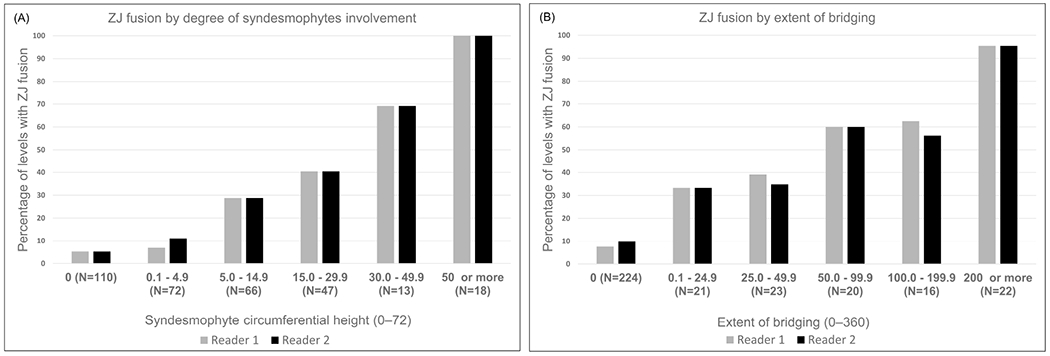
Frequency of ZJ fusion in either joint by (A) the magnitude of syndesmophyte circumferential height and (B) the extent of bridging syndesmophytes in each vertebral level. ZJ: zygapophyseal joint.
Association between ZJ fusion and bridging syndesmophytes.
The most common configuration [64%/62% of 326 levels (reader 1/reader 2)] was a vertebral level with no bridging syndesmophyte and no ZJ fusion, followed by vertebral levels with both bridging syndesmophytes and ZJ fusion (Table 2). However, there were twice as many vertebral levels with bridging but no ZJ fusion as there were levels with ZJ fusion but no bridging syndesmophytes. ZJ fusion was more common in levels with more extensive bridging (Figure 2B), but in vertebral levels with bridging spanning up to 50° of the vertebral rim, only 39%/35% (reader 1/reader 2) had ZJ fusion.
These results suggest that ZJ fusion tracks more closely with bridging syndesmophytes at the same vertebral level than with nonbridging syndesmophytes. This is supported by the finding that among 216 vertebral levels with any syndesmophyte (either bridging or not), 32%/34% (reader 1/reader 2) had ZJ fusion, while among 102 vertebral levels with bridging syndesmophytes, 58%/56% (reader 1/reader 2) had ZJ fusion.
Contribution of ZJ fusion, syndesmophytes, and bridging to lumbar motion.
The number of vertebral levels with ZJ fusion correlated inversely with the modified Schober test and lateral thoracolumbar flexion (Table 3). Correlations were similar with syndesmophyte circumferential height and extent of bridging syndesmophytes.
Table 3.
Correlations between no. vertebral levels with ZJ fusion, sum of syndesmophyte circumferential height among vertebral levels, and sum of extent of bridging syndesmophytes among vertebral levels and both the modified Schober test and lateral thoracolumbar flexion. The unit of analysis is the patient (n = 55). All p < 0.0001. Values are Spearman correlations.
| Variable | Modified Schober Test | Lateral Thoracolumbar Flexion |
|---|---|---|
| Levels with ZJ fusion, reader 1 | −0.60 | −0.66 |
| Levels with ZJ fusion, reader 2 | −0.63 | −0.65 |
| Syndesmophyte circumferential height | −0.61 | −0.64 |
| Extent of bridging | −0.62 | −0.66 |
ZJ: zygapophyseal joint.
We used multiple regression to test the relative association of ZJ fusion and each syndesmophyte measure with lumbar motion (Table 4). Each additional vertebral level with ZJ fusion was associated with a decrease in the modified Schober test of about 0.17 cm and in lateral thoracolumbar flexion of about 0.6 cm. Every 10-unit increase in circumferential syndesmophyte height was associated with a decrease in the modified Schober test of 0.06 cm and in lateral thoracolumbar flexion of about 0.3 cm. Based on the standardized β coefficients, which account for the differences in scale between these measures, ZJ fusion was slightly less strongly associated with the modified Schober test than were syndesmophytes, measured either by circumferential height or extent of bridging. Both syndesmophyte measures were more strongly associated with lateral thoracolumbar movement than was the number of vertebral levels with ZJ fusion.
Table 4.
Multiple regression analysis of the association of ZJ fusion, syndesmophytes, and bridging with lumbar motion. Results are based on ZJ fusion as seen by reader 1; results for reader 2 are present in Supplementary Table 1 (available from the authors on request).
| Model | Dependent Variable | Independent Variables | β (Standard Error) | Standardized β | p |
|---|---|---|---|---|---|
| 1 | Modified Schober test | ZJ fusion* | −0.172 (0.102) | −0.25 | 0.10 |
| Syndesmophyte circumferential height† | −0.060 (0.027) | −0.33 | 0.04 | ||
| BASDAI | −0.028 (0.014) | −0.23 | 0.05 | ||
|
| |||||
| 2 | Modified Schober test | ZJ fusion* | −0.184 (0.10) | −0.28 | 0.07 |
| Extent of bridging† | −0.012 (0.006) | −0.31 | 0.04 | ||
| BASDAI | −0.029 (0.014) | −0.23 | 0.04 | ||
|
| |||||
| 3 | Lateral thoracolumbar flexion | ZJ fusion* | −0.639 (0.384) | −0.23 | 0.11 |
| Syndesmophyte circumferential height† | −0.293 (0.10) | −0.40 | 0.005 | ||
| BASDAI | −0.164 (0.052) | −0.32 | 0.003 | ||
|
| |||||
| 4 | Lateral thoracolumbar flexion | ZJ fusion* | −0.726 (0.375) | −0.26 | 0.06 |
| Extent of bridging† | −0.061 (0.022) | −0.37 | 0.008 | ||
| BASDAI | −0.169 (0.052) | −0.32 | 0.002 | ||
Per additional vertebral level fused (of 6).
Per additional 10 units of syndesmophyte height or additional 10° of bridging among all 6 vertebral levels.
ZJ: zygapophyseal joint; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index.
Longitudinal study.
Thirty-one patients had a followup CT scan at Year 2 and 6 patients had a followup scan at Year 4. Among the 31 patients, there were 271 ZJ without fusion at baseline and able to demonstrate progression. Only 1 ZJ (0.4%) progressed to fusion over 2 years. In comparison, among 176 intervertebral disc spaces in the same patients that were not completely fused (i.e., where syndesmophyte height could still progress), 93 (53%) exhibited progression in syndesmophyte circumferential height.
The 6 patients with 4-year scans had a total of 46 ZJ without fusion at baseline. Only 1 ZJ (2.2%) progressed to fusion at 4 years. In the same patients, of 33 intervertebral disc spaces that were not completely fused at baseline, 30 (91%) exhibited progression in syndesmophyte circumferential height.
DISCUSSION
Although ZJ are commonly affected in AS, their potential contribution to spinal fusion has been overshadowed by a focus on the more-readily detected changes in the vertebral bodies and disc spaces15,16. For example, the modified Stoke AS Spine Score and different magnetic resonance imaging scores do not include ZJ abnormalities17,18. ZJ fusion is scored in the BASRI spine, but in this score it is not distinguished from anterior vertebral fusion13. CT overcomes many of the limitations of radiography, providing improved visualization of ZJ and potentially allowing more accurate readings. CT therefore allows investigating whether ZJ abnormalities could be a useful measure of spinal fusion in AS, particularly in comparison to syndesmophyte formation. To our knowledge, ours is the first study to investigate ZJ fusion and syndesmophytes simultaneously in patients with AS using CT scanning, and the first longitudinal study of ZJ fusion. Our results indicate that ZJ fusion does not provide a distinct advantage over syndesmophytes as a measure of spinal fusion in AS.
ZJ fusion in the thoracolumbar spine was reliably detected on CT scans, and results were closely similar between the 2 readers. However, there was less consistency between readers in distinguishing normal ZJ from those that were abnormal but not fused. This was due to limited resolution of the scans and varying interpretation of narrowing or irregularity in the small joint spaces. The moderate reliability of a 3-category grading of ZJ abnormalities is a limitation not shared by quantitative measurement of syndesmophytes9. ZJ fusion was present in 51% of patients, who had a mean duration of AS of 18.5 years. Because ZJ fusion partly reflects chronicity, this proportion would be expected to vary with disease duration. ZJ fusion occurs slowly, with joints rarely becoming fused even over 4 years. This rate contrasts with syndesmophyte growth in most patients over the same period11.
ZJ fusion was more common at the thoracolumbar junction than in the lower lumbar spine, as are syndesmophytes. However, syndesmophytes were often present at vertebral levels without ZJ fusion, while ZJ fusion was rarely present without syndesmophytes. This pattern suggests that syndesmophytes most often develop before ZJ fusion occurs in the same vertebral level. Structural spinal damage would therefore be detected earlier by syndesmophyte growth than by ZJ fusion in most cases. There was a greater concordance between ZJ fusion and bridging syndesmophytes than between ZJ fusion and syndesmophytes of any size, likely because both processes require time to develop. However, bridging without ZJ fusion at the same level was twice as common as ZJ fusion without bridging, suggesting that bridging more often precedes ZJ fusion than the converse. These results differ from those of de Vlam, et al, who concluded the ZJ fusion was the primary structural lesion in AS, based on the finding that ZJ fusion without bridging was a more common pattern8. The study used radiographs, which may have affected the accuracy of detection of ZJ fusion. Bridging syndesmophytes were scored only at the anterior vertebral borders on lateral films. Eleven percent of lumbar vertebral levels were reported to be bridged, in contrast to 31% in our study. Scoring only anterior vertebral bridging likely underestimated the presence of bridging, which most often occurs at the lateral or posterolateral vertebral rim19. Differences in the vertebral levels examined (T12–L1 to L5–S1 vs T10–T11 to L3–L4 in our study) may have also contributed to discordant results because syndesmophytes and bridging are more common in the lower thoracic spine.
ZJ fusion was associated with restricted lumbar motion. Our study extends the results of 2 small previous reports by demonstrating that ZJ fusion was only marginally associated with restricted motion after considering the contribution of syndesmophytes and AS activity6,7. Bridging and ZJ fusion were similarly associated with limitations in lumbar forward flexion, while bridging was more strongly associated with limited lateral flexion. The predominantly vertical orientation of the lumbar ZJ joints in the sagittal plane may account for the stronger association of ZJ fusion with limited forward flexion20,21. These results indicate that while ZJ fusion may contribute to spinal rigidity, it is not a more prominent determinant of lumbar motion than bridging syndesmophytes. Although spinal motion is also affected by symptoms and inflammation, the BASDAI in our cohort was low, and adjustment for the BASDAI did not alter the associations between the radiographic features and spinal motion22,23.
Our cohort of 55 patients is relatively large compared with previous CT studies of ZJ joints, but is still modest in size. However, it was large enough to determine associations with lumbar motion and differences in ZJ fusion by vertebral level. Few levels had ZJ fusion but no syndesmophytes, which might have limited our ability to detect associations with ZJ fusion specifically. Our study is also limited by the lack of finer gradation in ZJ scoring. This limited our ability to study associations with joint abnormalities other than fusion. We do not know whether syndesmophytes are associated with ZJ damage other than fusion. Quantitation of ZJ narrowing might provide greater accuracy, but would require extensive technical development, and may not be feasible in the smaller thoracic ZJ. We also examined only 6 vertebral levels. We do not know whether similar associations would be present in the cervical or upper thoracic spine. In the future, the range could be extended as new scanner technology allows for reductions in radiation dose with preserved image quality. Last, we did not obtain lumbar oblique films, and so could not compare plain radiography with CT for detection of ZJ fusion.
Thoracolumbar ZJ fusion is common in AS and is an important contributor to restricted spinal motion. However, based on their higher prevalence, earlier appearance, greater rates of change, and associations with lumbar motion, syndesmophytes are a more useful imaging marker of spinal fusion in AS.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program, US National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant number ZIA-AR-041153); by the NIH Clinical Center; and the Johns Hopkins University School of Medicine General Clinical Research Center (grant number M01-RR00052 from the National Center for Research Resources/NIH).
Contributor Information
Sovira Tan, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Jianhua Yao, Radiology and Imaging Sciences, Clinical Center, NIH.
John A. Flynn, Division of Rheumatology, Johns Hopkins Medical Institutions
Lawrence Yao, Radiology and Imaging Sciences, Clinical Center, NIH.
Michael M. Ward, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH
REFERENCES
- 1.Appel H, Kuhne M, Spiekermann S, Ebhardt H, Grozdanovic Z, Köhler D, et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum 2006;54:2845–51. [DOI] [PubMed] [Google Scholar]
- 2.Bleil J, Maier R, Hempfing A, Schlichting U, Appel H, Sieper J, et al. Histomorphologic and histomorphometric characteristics of zygapophyseal joint remodeling in ankylosing spondylitis. Arthritis Rheumatol 2014;66:1745–54. [DOI] [PubMed] [Google Scholar]
- 3.Varlotta GP, Lefkowitz TR, Schweitzer M, Errico TJ, Spivak J, Bendo JA, et al. The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal Radiol 2011;40:13–23. [DOI] [PubMed] [Google Scholar]
- 4.Kettler A, Wilke HJ. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J 2006;15:705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubrano E, Marchesoni A, Olivieri I, D’Angelo S, Spadaro A, Parsons WJ, et al. Psoriatic arthritis spondylitis radiology index: a modified index for radiologic assessment of axial involvement in psoriatic arthritis. J Rheumatol 2009;36:1006–11. [DOI] [PubMed] [Google Scholar]
- 6.Simkin PA, Downey DJ, Kilcoyne RF. Apophyseal arthritis limits lumbar motion in patients with ankylosing spondylitis. Arthritis Rheum 1988;31:798–802. [DOI] [PubMed] [Google Scholar]
- 7.Russell AS, Jackson F. Computer assisted tomography of the apophyseal changes in patients with ankylosing spondylitis. J Rheumatol 1986;13:581–5. [PubMed] [Google Scholar]
- 8.de Vlam K, Mielants H, Veys EM. Involvement of the zygapophyseal joint in ankylosing spondylitis: relation to the bridging syndesmophyte. J Rheumatol 1999;26:1738–45. [PubMed] [Google Scholar]
- 9.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative measurement of syndesmophyte volume and height in ankylosing spondylitis using CT. Ann Rheum Dis 2014;73:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitation of circumferential syndesmophyte height along the vertebral rim in ankylosing spondylitis using computed tomography. J Rheumatol 2015;42:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis 2015;74:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 13.MacKay K, Mack C, Brophy S, Calin A. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum 1998;41:2263–70. [DOI] [PubMed] [Google Scholar]
- 14.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 15.Lee S, Lee JY, Hwang JH, Shin JH, Kim TH, Kim SK. Clinical importance of inflammatory facet joints of the spine in ankylosing spondylitis: a magnetic resonance imaging study. Scand J Rheumatol 2016;45:491–8. [DOI] [PubMed] [Google Scholar]
- 16.Maksymowych WP, Crowther SM, Dhillon SS, Conner-Spady B, Lambert RG. Systematic assessment of inflammation by magnetic resonance imaging in the posterior elements of the spine in ankylosing spondylitis. Arthritis Care Res 2010;62:4–10. [DOI] [PubMed] [Google Scholar]
- 17.Wanders AJ, Landewé RB, Spoorenberg A, Dougados M, van der Linden S, Mielants H, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004;50:2622–32. [DOI] [PubMed] [Google Scholar]
- 18.Lukas C, Braun J, van der Heijde D, Hermann KG, Rudwaleit M, Østergaard M, et al. ; ASAS/OMERACT MRI in AS Working Group. Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 2007;34:862–70. [PubMed] [Google Scholar]
- 19.Tan S, Dasgupta A, Yao J, Flynn JA, Yao L, Ward MM. Spatial distribution of syndesmophytes along the vertebral rim in ankylosing spondylitis: preferential involvement of the posterolateral rim. Ann Rheum Dis 2016;75:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaumard NV, Welch WC, Winkelstein BA. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J Biomech Eng 2011;133:071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozanek M, Wang S, Passias PG, Xia Q, Li G, Bono CM, et al. Range of motion and orientation of the lumbar facet joints in vivo. Spine 2009;34:E689–96. [DOI] [PubMed] [Google Scholar]
- 22.Machado P, Landewé R, Braun J, Hermann KG, Baker D, van der Heijde D. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. [DOI] [PubMed] [Google Scholar]
- 23.Calvo-Gutierrez J, Garrido-Castro JL, Gil-Cabezas J, Gonzalez-Navas C, Ugalde PF, Carmona L, et al. Is spinal mobility in patients with spondylitis determined by age, structural damage, and inflammation? Arthritis Care Res 2015;67:74–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


