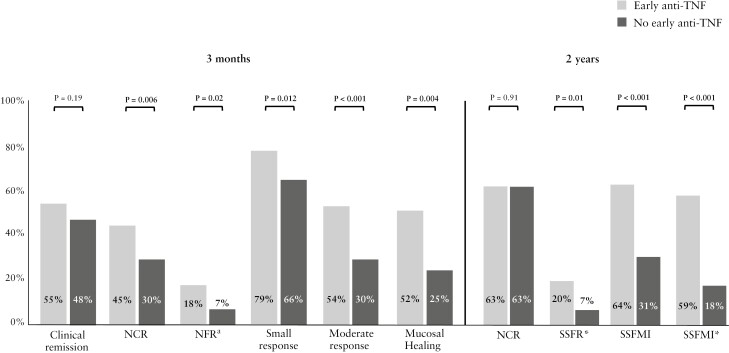
Figure 2.
Treatment targets at 3 months and 2 years in children with Crohn’s disease, stratified by early anti-TNF use. aThese outcomes were only evaluated in patients with available faecal calprotectin results. bMucosal healing was estimated by the Mucosal Inflammation Noninvasive [MINI] Index. TNF, tumour necrosis factor; C-reactive protein; FCP, faecal calprotectin; NCR, normal CRP remission [remission with CRP <0.5 mg/dl]; NFR, normal FCP remission [remission with faecal calprotectin <250 μg/g]; SSFR*, sustained steroid-free remission without treatment intensification; SSFMI, sustained steroid-free mild or inactive disease; SSFMI*, SSFMI without treatment intensification.