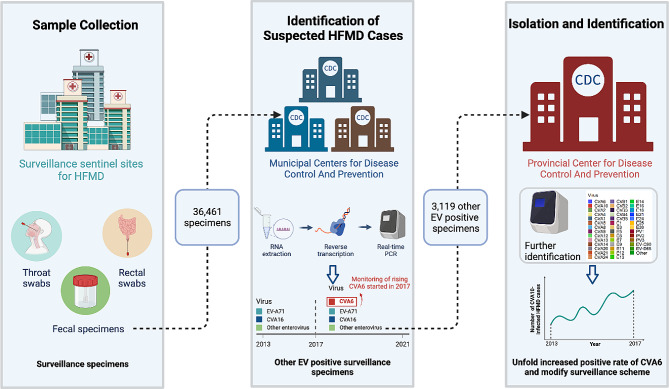
Fig. 1.
Workflow of the Guangdong Provincial HFMD surveillance Network. Medical institutions were responsible for reporting clinical cases and collecting specimens. Twenty-one Municipal Centers for Disease Control (CDCs) collected anal swabs or stool samples weekly from suspected HFMD cases and carried out primary molecular testing. From 2013 to 2016, RT-PCR testing was undertaken to detect enteroviruses, EVA71 and CVA16, and CVA6 targeted testing had been included since 2017. The specimens that tested positive for enteroviruses but negative for EVA71 and CVA16 from the years 2013–2016, as well as those negative for CVA6 from 2017–2021 were sent to the provincial CDC for further virus isolation and genetic sequencing