ABSTRACT
Background: In recent years, infectious diseases like COVID-19 have had profound global socio-economic impacts. mRNA vaccines have gained prominence due to their rapid development, industrial adaptability, simplicity, and responsiveness to new variants. Notably, the 2023 Nobel Prize in Physiology or Medicine recognized significant contributions to mRNA vaccine research. Methods: Our study employed a comprehensive bibliometric analysis using the Web of Science Core Collection (WoSCC) database, encompassing 5,512 papers on mRNA vaccines from 2003 to 2023. We generated cooperation maps, co-citation analyses, and keyword clustering to evaluate the field’s developmental history and achievements. Results: The analysis yielded knowledge maps highlighting countries/institutions, influential authors, frequently published and highly cited journals, and seminal references. Ongoing research hotspots encompass immune responses, stability enhancement, applications in cancer prevention and treatment, and combating infectious diseases using mRNA technology. Conclusions: mRNA vaccines represent a transformative development in infectious disease prevention. This study provides insights into the field’s growth and identifies key research priorities, facilitating advancements in vaccine technology and addressing future challenges.
KEYWORDS: mRNA vaccine, bibliometric analysis, hotspot, 2023 Nobel prize
Introduction
Vaccination is currently the most effective and economical intervention for controlling the spread of infectious diseases and reducing their impact. Globally, routine vaccination rates have now exceeded 80%.1 Compared to traditional vaccines, mRNA vaccines offer unique advantages in preventing infectious diseases due to their flexibility in development and high immunogenicity.2,3 Significant achievements have been made in the field of mRNA vaccines, including in the treatment and prevention of tumors, control of infectious diseases, and innate immune therapy.4,5 In 2023, the Nobel Prize in Physiology and Medicine was awarded to Hungarian-American scientist Katalin Karikó and American scientist Drew Weissman from the University of Pennsylvania for their outstanding contributions to mRNA vaccine research.
The most heated discussion regarding mRNA vaccines undeniably centers around the COVID-19 mRNA vaccines. Following the outbreak in 2019, Pfizer-BioNTech and Moderna from the United States were the first to introduce two mRNA vaccines, Comirnaty and Spikevax. Subsequently, several other mRNA vaccines were developed, contributing significantly to the fight against the COVID-19 pandemic and aiding in the development of herd immunity.6 Therefore, exploring the historical research trends of mRNA vaccines, changes in research hotspots, the underlying reasons for these shifts, discussing how mRNA moved from basic research to clinical vaccine development and shone in the field of COVID-19 vaccines, as well as the prospects of mRNA vaccines in treating more infectious diseases and tumors, are questions of great practical significance. Furthermore, how to visually and intuitively represent the contributions of Katalin Karikó and Drew Weissman in the development of mRNA vaccines has also sparked considerable curiosity.
To better address these questions and determine future research directions, we have introduced bibliometric analysis, a method for studying academic publications that reveals the current state of research in a specific field.7 First proposed in 1969, bibliometric analysis has evolved into an interdisciplinary field encompassing bibliography, statistics, and mathematics. Its characteristics include calculating and visualizing the contributions of different countries, authors, and journals to a given field.8,9 Tools like CiteSpace and VOSviewer can automatically generate interactive visual networks from scientific publication records for bibliometric analysis.10 Due to its powerful analytical and evaluative capabilities, bibliometric analysis is widely used in medical research fields such as oncology, immunology, and public health.11,12–15
This study, based on the bibliometric analysis of literature from the Web of Science Core Collection (WoSCC), aims to: (1) Discover research trends in mRNA vaccines over the past 20 years; (2) Focus on the contributions of Nobel Prize winners and their institutions in this field and visualize them; (3) Identify challenging questions and research hotspots related to mRNA vaccines; (4) Construct a knowledge map of the field; (5) Provide valuable insights for future related research.
Materials and methods
Data was sourced from the Web of Science Core Collection (WoSCC) database, covering the period from January 1, 2003, to October 15, 2023. We conducted searches for records related to “mRNA vaccines,” “mRNA vaccines and lipid nanoparticles,” and “nucleoside and base modifications.” The search strategy was: TS=((“mRNA Vaccine”) OR (“mRNA Vaccines”) OR (“mRNA Vaccination”) OR (“RNA Vaccination”) OR (“RNA Vaccine”)). We limited document types to articles and reviews without language restrictions. All searches were completed on October 15, 2023, and the records were saved as plain text files, named download_txt.
The emergence of mRNA vaccines and their widespread use during the COVID-19 pandemic have underscored the critical roles of nucleoside/base modifications and lipid nanoparticles. The 2023 Nobel Prize in Physiology and Medicine was awarded to two scientists for their contributions in these areas. To further explore the contributions of Nobel laureates Weissman D and Kariko K in the field of mRNA vaccines, we separately searched for literature related to lipid nanoparticles and base modifications, conducting visual analyses of authors and institutions. The search strategies were: (TS=((“lipid nanoparticles”) OR (“lipid nanoparticle”) OR (“solid lipids nanoparticle”) OR (“solid lipid nanoparticle”))) AND TS=(“mRNA vaccines”), (TS=(“nucleoside modification”)) OR TS=(“base modification”)). We limited document types to articles and reviews without language restrictions. All searches were completed on October 15, 2023, and the records were saved as plain text files, named download_txt.
The aforementioned data were imported into CiteSpace6.2.R4, VOSviewer1.6.19, and Bibliometrix R Package software. CiteSpace6.2.R4 was used for country/region analysis, institutional analysis, co-cited author analysis, journal co-citation overlay, literature co-citation and cluster analysis, and keyword and emerging trend analysis. VOSviewer 1.6.19 was employed for country/region analysis, institutional analysis, author and co-cited author analysis, journal co-citation analysis, and keyword and cluster analysis. The Bibliometrix R Package software was utilized for institutional analysis, journal analysis, and thematic trend analysis.
Results
Annual publication output in the field of mRNA vaccines
Based on the search process diagram (Figure 1A), from 2003 to 2023, a total of 4,406 publications related to mRNA vaccines were published, with 3,598 articles (81.66%) and 808 reviews (18.34%).
Figure 1.
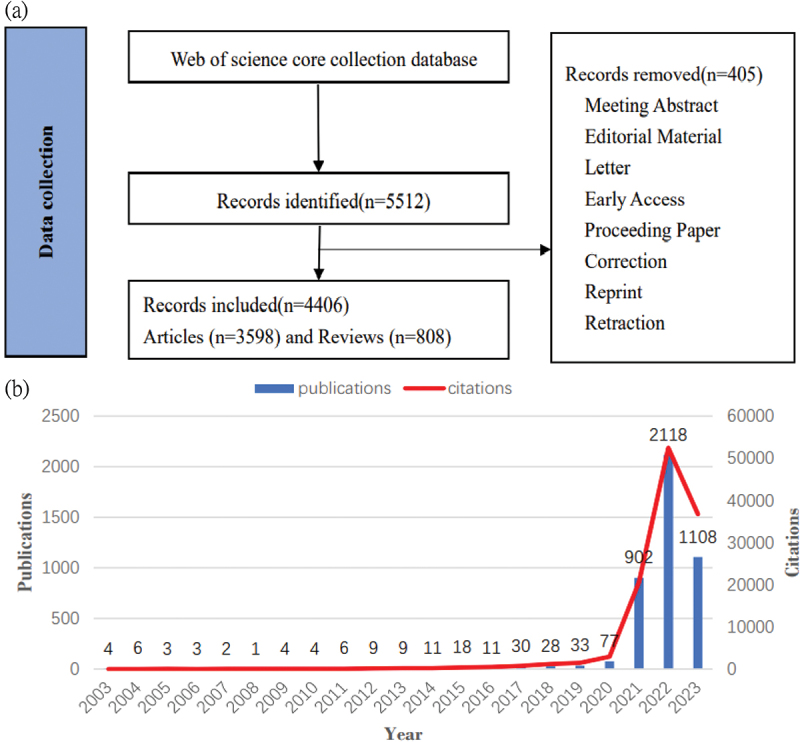
(a) Flowchart of the literature searching and screening in the study. (b) Global trend of publications and citations on mRNA vaccine.
The dynamic change in the number of publications over the past decade reflects the overall development trend in this field. As shown in (Figure 1b), the output of publications in the mRNA field was on a slow upward trend from 2003 to 2019, with a very slight annual increase. However, a significant growth in the number of publications and citations was observed from 2019 to 2020, with the increase exceeding the total of the previous plateau period, followed by exponential growth from 2020 to 2022. In 2023, instead of a further increase, there was a substantial decline, with the number of publications and total citations dropping from 2,118 and 52,472 in 2022 to 1,108 and 36,761 in 2023, respectively.
Contribution by country in the field of mRNA vaccines
In the world map distribution by Bibliometrix(Figure 2C), countries with a darker color contributed more in this field.16 Over twenty years, a total of 120 countries have conducted research on mRNA vaccines (Figure 2A). The United States led with 1,368 publications, followed by China with 487, and Italy with 454 (Table 1). North America (USA, Canada), Europe (Italy, Germany, England, etc.), and Asia (China, Japan) made significant contributions, while most other countries published fewer than 10 articles, and some regions are still void in this field.
Figure 2.
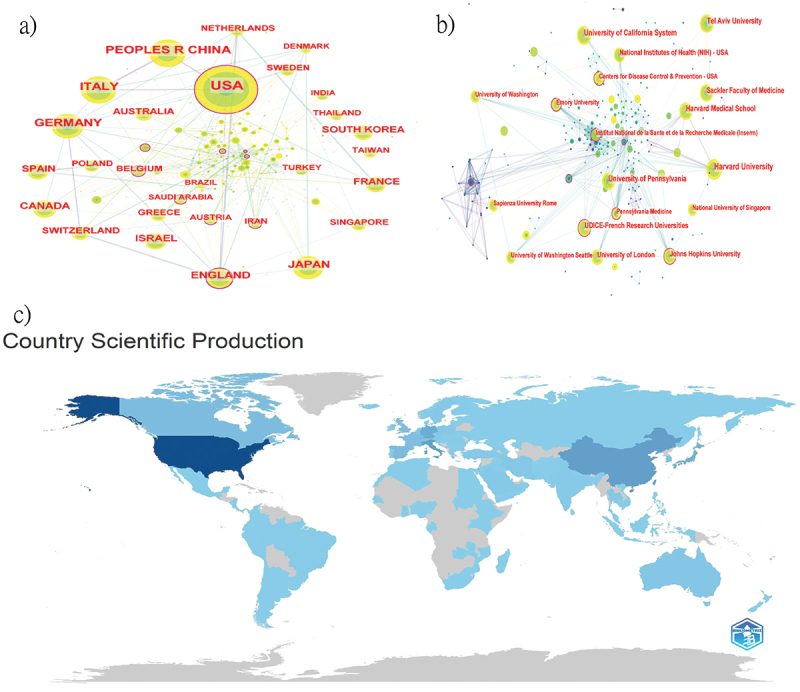
The collaboration of countries/institutions in the field of mRNA vaccine. (a-b) Co-occurrence network of countries/institutions. (c) Geographical distribution of global publications.
Table 1.
TOP10 productive countries and institutions regarding the research on application of lipid nanoparticles in mRNA vaccines.
| Rank | Country | Centrality | Count | ACI | H-index | Institution | Centrality | Count | ACI | H-index |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | USA | 0.07 | 1368 | 50.22 | 115 | HARVARD UNIVERSITY | 0.04 | 160 | 67.53 | 42 |
| 2 | China | 0.00 | 487 | 17.29 | 47 | UNIVERSITY OF CALIFORNIA SYSTEM | 0.01 | 121 | 37.25 | 29 |
| 3 | Italy | 0.23 | 454 | 14.73 | 40 | HARVARD MEDICAL SCHOOL | 0.00 | 115 | 65.64 | 33 |
| 4 | Germany | 0.06 | 404 | 65.55 | 63 | TEL AVIV UNIVERSITY | 0.00 | 112 | 62.38 | 37 |
| 5 | Japan | 0.00 | 322 | 8.11 | 20 | UNIVERSITY OF PENNSYLVANIA | 0.02 | 107 | 75.05 | 36 |
| 6 | England | 0.10 | 246 | 94.01 | 54 | SACKLER FACULTY OF MEDICINE | 0.00 | 103 | 61.52 | 34 |
| 7 | Canada | 0.07 | 190 | 28.74 | 36 | UDICE FRENCH RESEARCH UNIVERSITIES | 0.01 | 95 | 23.41 | 23 |
| 8 | Israel | 0.06 | 164 | 66.23 | 45 | JOHNS HOPKINS UNIVERSITY | 0.00 | 92 | 127.99 | 30 |
| 9 | Spain | 0.04 | 161 | 10.13 | 23 | NATIONAL INSTITUTES OF HEALTH NIH USA | 0.00 | 88 | 88.66 | 34 |
| 10 | France | 0.30 | 158 | 20.56 | 30 | UNIVERSITY OF LONDON | 0.03 | 87 | 67.85 | 33 |
ACI, average citations per item; USA, the United States of America.
In the Citespace diagram (Figure 2A), circles represent countries; the size of the circle indicates the number of publications; lines between circles represent collaborative relationships. The purple outer ring in Citespace highlights the intermediacy centrality, emphasizing its importance in the network of international collaboration. China and Japan, despite having a significant number of articles, had less collaboration with other countries and lower intermediacy centrality. In contrast, most European countries had fewer articles but more international collaborations, leading to higher centrality. This may be due to earlier involvement in relevant research and frequent collaboration among institutions in Europe and America.
From 2003 to 2023, over four hundred institutions conducted research on mRNA (Figure 2B, Table 1). The most prolific was HARVARD UNIVERSITY (160 publications), followed by the UNIVERSITY OF CALIFORNIA SYSTEM (121 publications) and HARVARD MEDICAL SCHOOL (115 publications). Most of the top ten institutions are located in the USA. Notably, the University of Pennsylvania, affiliated with Nobel laureates, ranked fifth with 107 publications. Institutions in North America and Europe had larger nodes and more interconnections, indicating close collaboration; no Asian institutions appeared among the top ten in terms of output.
Authors and co-cited authors in the field of mRNA vaccines
Over the past twenty years, a total of 6,021 authors participated in mRNA vaccine research. Details of the top ten most productive authors are listed in (Table 2). The most published author was Nobel laureate Weissman Drew (41 publications), followed by Pardi Norbert from the University of Pennsylvania (38 publications) and Sahin Ugur from Suleyman Demirel University (36 publications). Based on the level of collaboration among the top 50 most productive authors, their cooperation was divided into six clusters (Figure 3A). The top ten most co-cited authors are also listed in (Table 2), with Sahin Ugur from Suleyman Demirel University having the highest number of co-citations (3,203). Interestingly, eight of the top ten most co-cited authors are from Pfizer, as shown in the co-citation cooperation network (Figure 3B).
Table 2.
Top 10 productive authors and co-cited authors in the field of mRNA vaccine.
| Rank | Author | Institution | Documents | ACI | H-index | Co-cited Author | Institution | Co-citations | H-index |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Weissman, Drew | University of Pennsylvania | 41 | 120.12 | 23 | Sahin, Ugur | Suleyman Demirel University | 3203 | 24 |
| 2 | Pardi, Norbert | University of Pennsylvania | 38 | 115.03 | 21 | Tuereci, Oezlem | Tuereci, Oezlem | 2940 | 17 |
| 3 | Sahin, Ugur | Suleyman Demirel University | 36 | 421.97 | 24 | Dormitzer, P. R. | Pfizer | 2876 | 10 |
| 4 | Gaglani, Manjusha | Baylor Scott and White Health | 32 | 50.53 | 14 | Swanson, Kena A. | Pfizer | 2676 | 7 |
| 5 | Krammer, Florian | Icahn School of Medicine at Mount Sinai | 27 | 69.07 | 16 | Cooper, David | Pfizer | 2623 | 7 |
| 6 | Tenforde, M. W. | Centers for Disease Control & Prevention | 24 | 45.33 | 11 | Jansen, Kathrin U. | Pfizer | 2609 | 6 |
| 7 | Tam, Ying K | Acuitas Therapeut | 22 | 83.05 | 13 | Absalon, J. | Pfizer | 2303 | 4 |
| 8 | Muramatsu, Hiromi | University of Pennsylvania | 21 | 92.71 | 13 | Bailey, Ruth | Pfizer | 2303 | 4 |
| 9 | Agrati, Chiara | Natl Inst Infect Dis | 21 | 25.48 | 12 | Gruber, William C. | Pfizer | 2303 | 4 |
| 10 | Alter, G. | MIT & Harvard, Ragon Inst MGH, Cambridge | 21 | 38.38 | 10 | Gurtman, Alejandra | Pfizer | 2303 | 4 |
Figure 3.
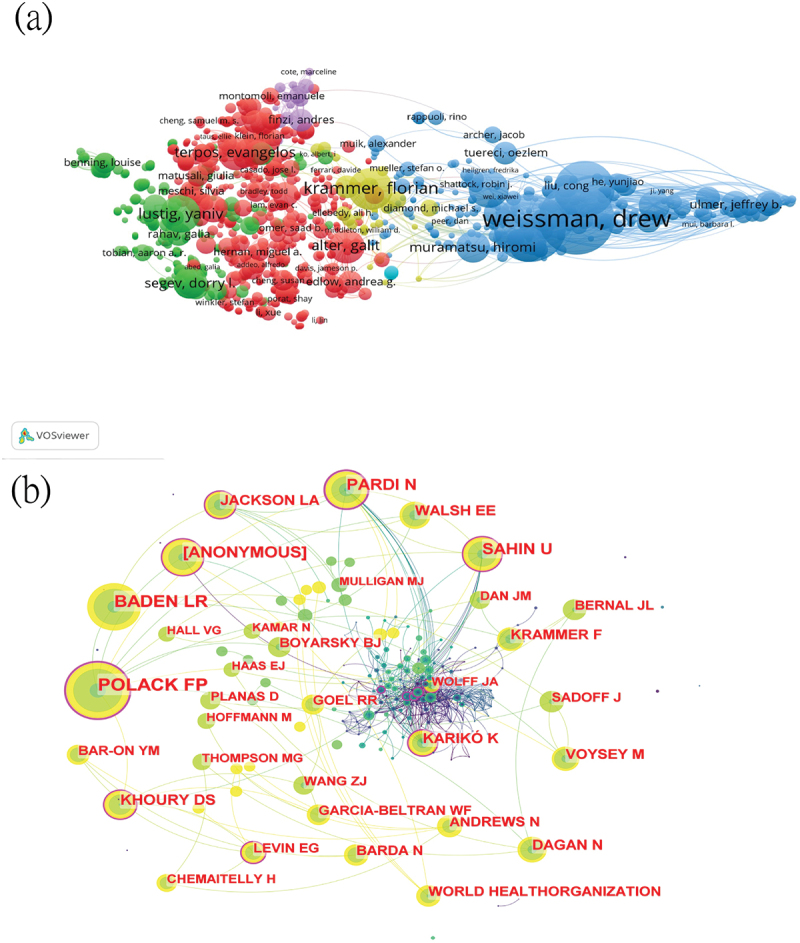
The collaboration of authors and co-cited authors in the field of mRNA vaccine. (a) Cooperation network among the authors. (b) Cooperation network among the co-cited authors.
Contributions of Nobel laureates
In the field of base modification, the co-occurrence graph reveals dense connections and numerous adjacent nodes for scientists Weissman D and Kariko K (Figure 4A). The University of Pennsylvania ranks eighth among over 100 institutions worldwide researching this field (Figure 4B), and its intermediacy centrality and ACI (average citations per item), which indicate the importance and quality of output, rank third and second among the top ten institutions by output quantity (Table 3). In the application of lipid nanoparticles in mRNA vaccines, as shown in (Table 4), scholar Weissman D ranks first in output quantity (19 publications), with scholar Kariko K also among the top ten most prolific authors (9th, previously 6th), exhibiting higher intermediacy centrality (0.01). In (Figure 5A), nodes surrounding Weissman D and Kariko K among the top ten most productive scholars have more adjacent nodes and connections. In the co-cited author diagram (Figure Figure 5B), Kariko K ranks fourth in co-citations (107) and first in intermediacy centrality (0.16). The University of Pennsylvania and its Medical School lead with 27 publications(Figure 5C, Table 5), ranking first in both output and intermediacy centrality (0.16). Notably, among the top 10 most prolific authors, the publications by Langer, Robert exhibited the highest ACI, indicating that Langer’s research in this field has been exceptionally valuable and widely cited by peers.
Figure 4.
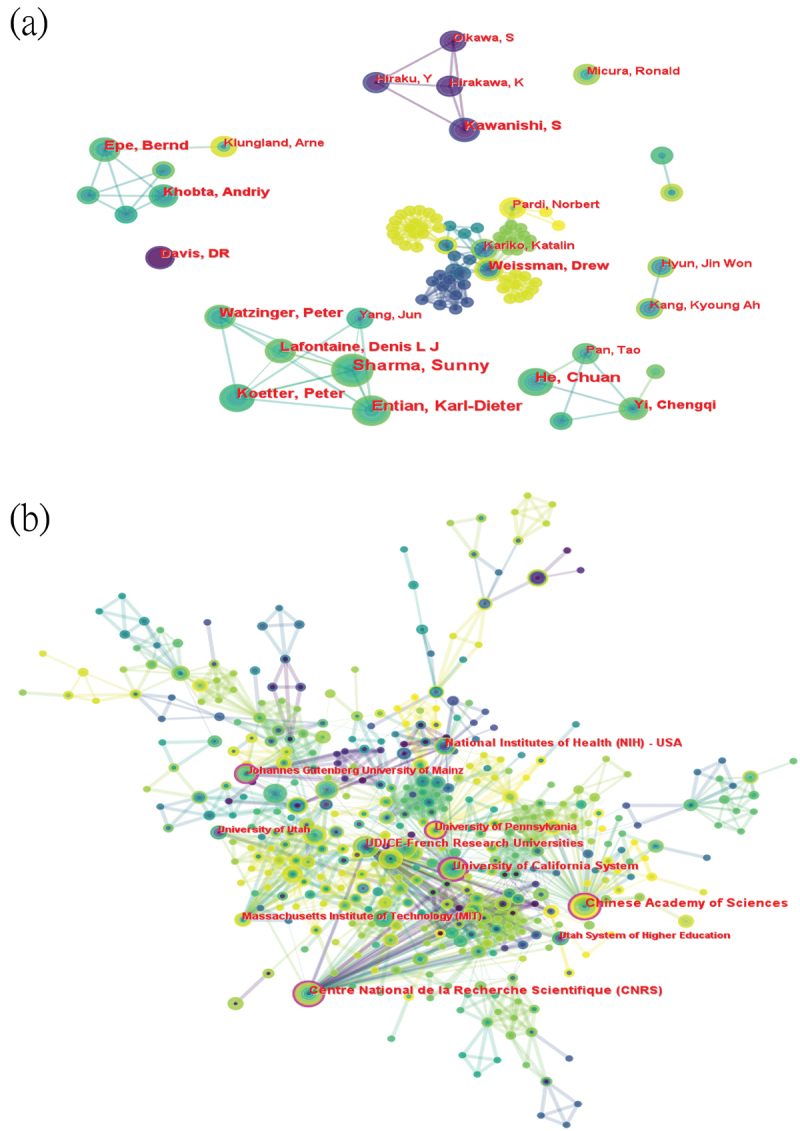
The collaboration of authors and institutions in the field of base modification in mRNA vaccines. (a) Cooperation network among the authors. (b) Co-occurrence network of institutions.
Table 3.
TOP10 productive institutions regarding the research on base modification.
| Rank | Count | Centrality | Year | Instiution | H-index | ACI |
|---|---|---|---|---|---|---|
| 1 | 21 | 0.12 | 2006 | Chinese Academy of Sciences | 11 | 76.33 |
| 2 | 20 | 0.13 | 2000 | Centre National de la Recherche Scientifique (CNRS) | 11 | 59.75 |
| 3 | 16 | 0.06 | 2000 | UDICE-French Research Universities | 10 | 48.88 |
| 4 | 14 | 0.24 | 2001 | University of California System | 11 | 152.79 |
| 5 | 13 | 0.08 | 2000 | National Institutes of Health (NIH) – USA | 12 | 41.15 |
| 6 | 11 | 0.08 | 2000 | Massachusetts Institute of Technology (MIT) | 8 | 82.64 |
| 7 | 11 | 0.11 | 2003 | Johannes Gutenberg University of Mainz | 10 | 72.18 |
| 8 | 11 | 0.12 | 2005 | University of Pennsylvania | 10 | 231.18 |
| 9 | 10 | 0 | 2000 | University of Utah | 9 | 27.2 |
| 10 | 9 | 0 | 2010 | University of Chicago | 9 | 303.67 |
Table 4.
TOP10 co-citations and productive author regarding the research on application of lipid nanoparticles in mRNA vaccines.
| Rank | Author | Co-citations | Centrality | Year | Author | Count | Centrality | ACI | Year |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pardi N | 179 | 0.13 | 2017 | Weissman, Drew | 19 | 0 | 124.47 | 2018 |
| 2 | Sahin U | 124 | 0.09 | 2017 | Pardi, Norbert | 16 | 0 | 91.83 | 2018 |
| 3 | Polack Fp | 108 | 0.03 | 2021 | Tam, Ying K | 11 | 0.01 | 85.56 | 2017 |
| 4 | Karikó K | 107 | 0.16 | 2017 | Muramatsu, Hiromi | 10 | 0 | 90.25 | 2018 |
| 5 | Baden Lr | 90 | 0.01 | 2021 | Ciaramella, Giuseppe | 8 | 0.01 | 248.5 | 2017 |
| 6 | Hassett Kj | 83 | 0.03 | 2021 | Yuzhakov, Olga | 7 | 0 | 191.43 | 2017 |
| 7 | Hou Xc | 79 | 0.03 | 2021 | Song, Xiangrong | 7 | 0 | 31.5 | 2021 |
| 8 | Akinc A | 76 | 0.06 | 2020 | Tombacz, Istvan | 7 | 0 | 144.83 | 2018 |
| 9 | Richner Jm | 75 | 0.07 | 2017 | Kariko, Katalin | 6 | 0.01 | 152.67 | 2018 |
| 10 | Kowalski Ps | 70 | 0.06 | 2020 | Langer, Robert | 6 | 0 | 297.83 | 2017 |
ACI, average citations per item.
Figure 5.
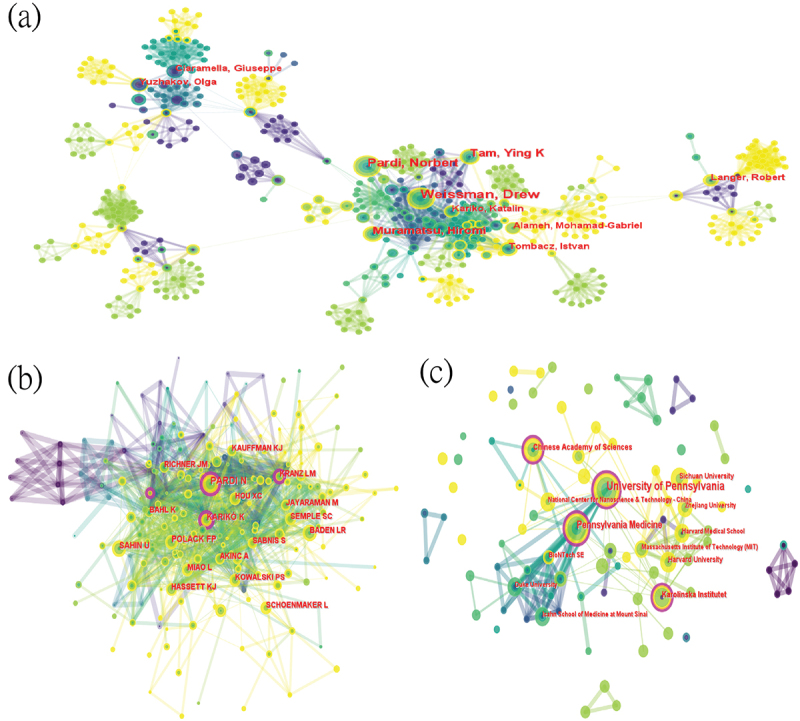
The collaboration of authors and institutions in the field of lipid nanoparticles in mRNA vaccines. (a) Cooperation network among the authors. (b) Cooperation network among the co-cited authors. (c) Co-occurrence network of institutions.
Table 5.
TOP10 productive institutions regarding the research on application of lipid nanoparticles in mRNA vaccines.
| Rank | Count | Centrality | Year | Institution |
|---|---|---|---|---|
| 1 | 27 | 0.16 | 2018 | University of Pennsylvania |
| 2 | 19 | 0.12 | 2018 | Pennsylvania Medicine |
| 3 | 10 | 0.2 | 2017 | Karolinska Institutet |
| 4 | 10 | 0.11 | 2020 | Chinese Academy of Sciences |
| 5 | 9 | 0.01 | 2022 | Sichuan University |
| 6 | 8 | 0.08 | 2017 | Harvard University |
| 7 | 8 | 0 | 2018 | BioNTech SE |
| 8 | 7 | 0.07 | 2017 | Massachusetts Institute of Technology (MIT) |
| 9 | 7 | 0.07 | 2020 | National Center for Nanoscience & Technology – China |
| 10 | 6 | 0.15 | 2021 | Centre National de la Recherche Scientifique (CNRS) |
Journals and co-citations in the field of mRNA vaccines
A total of 991 journals reported on mRNA vaccine research, with the top ten listed in (Table 6). Vaccines is the most prolific journal (444 publications, 10.08%), followed by Frontiers in Immunology (224 publications, 5.08%) and Vaccine (133 publications, 3.02%). Among the top ten, five are from the UK, four from Switzerland, and one from the USA. Seven journals have an impact factor > 5, including Vaccines (7.8), Frontiers in Immunology (7.3), Vaccine (5.5), Clinical Infectious Diseases (11.8), Nature Communications (16.6), Journal of Infectious Diseases (6.4), and International Journal of Molecular Sciences (5.6).
Table 6.
Top 10 productive journals in the field of mRNA vaccine.
| Rank | Journal | Publications | Country | Impact factor (2022) | SCImago Journal Rank(2022) | JCR | H-index |
|---|---|---|---|---|---|---|---|
| 1 | Vaccines | 444 | Switzerland | 7.8 | 1.655 | Q1 | 36 |
| 2 | Frontiers in Immunology | 224 | Switzerland | 7.3 | 2.022 | Q1 | 24 |
| 3 | Vaccine | 133 | United Kingdom | 5.5 | 1.493 | Q2 | 21 |
| 4 | Viruses-Basel | 68 | Switzerland | 4.7 | NA | Q2 | 10 |
| 5 | Clinical Infectious Diseases | 63 | United Kingdom | 11.8 | 3.995 | Q1 | 18 |
| 6 | Nature Communications | 61 | United Kingdom | 16.6 | 5.116 | Q1 | 22 |
| 7 | Human Vaccines Immunotherapeutics | 51 | United States | 4.8 | 1.041 | Q1 | 9 |
| 8 | Journal of Infectious Diseases | 45 | United Kingdom | 6.4 | 2.386 | Q1 | 13 |
| 9 | Scientific Reports | 39 | United Kingdom | 4.6 | 0.973 | Q2 | 9 |
| 10 | International Journal of Molecular Sciences | 37 | Switzerland | 5.6 | 1.154 | Q1 | 7 |
JCR, journal citation reports.
In a given research field, the frequency of journal citations greatly reflects its influence. This study used VOSviewer for co-citation analysis (Figure 6A). Each node represents a journal; the size of the node indicates the number of citations; lines between nodes represent co-citation relationships. The results show that journals can be divided into three main clusters: red representing clinically-oriented journals such as the New England Journal of Medicine and The Lancet; blue representing basic medical research journals such as Nature and Cell; and green representing interdisciplinary journals (medical-bioengineering, medical-chemistry, etc.) such as Journal of Controlled Release and Molecular Therapy. The New England Journal of Medicine is the most influential journal in this field, with 12,636 citations, demonstrating its high authority in mRNA vaccines. Among the top 10 most-cited journals, six have an impact factor > 50, and eight are Q1, indicating the high quality of articles related to mRNA vaccines and the value of this study (Table 7).
Figure 6.
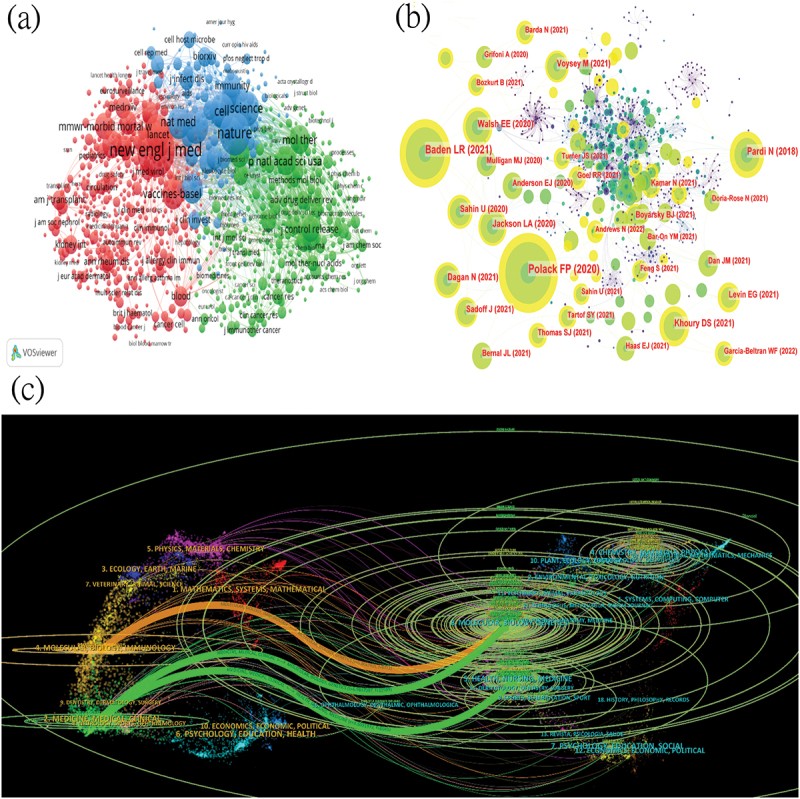
Visualization of the cited journals, co-citations journal, co-cited reference, and co-cited author analysis. (a) Co-occurrence network of cited journals. (b) Co-occurrence network of co-cited authors. (c) The dual-map overlay of articles citing mRNA vaccines. (The left side represents the citing journal, the right side represents the cited journal, and the lines indicate citation relationships).
Table 7.
Top 10 co-cited journals in the field of mRNA vaccine.
| Rank | Journal | Citations | Country | Impact Factor (2022) | SCImago Journal Rank(2022) | JCR | H-index |
|---|---|---|---|---|---|---|---|
| 1 | New England Journal of Medicine | 12636 | United States | 158.5 | 26.015 | Q1 | 22 |
| 2 | Nature | 7408 | United Kingdom | 64.8 | 20.957 | Q1 | 23 |
| 3 | Science | 4504 | United States | 56.9 | 13.328 | Q1 | 10 |
| 4 | Lancet | 4423 | United Kingdom | 168.9 | 14.607 | Q1 | 11 |
| 5 | Cell | 4151 | United States | 64.5 | 26.494 | Q1 | 14 |
| 6 | Vaccine | 4125 | United Kingdom | 5.5 | 1.493 | Q2 | 21 |
| 7 | Nature Medicine | 3621 | United Kingdom | 82.9 | 24.678 | Q1 | 18 |
| 8 | Viruses-Basel | 3257 | Switzerland | 4.7 | NA | Q2 | 10 |
| 9 | Frontiers in Immunology | 3027 | Switzerland | 7.3 | 2.022 | Q1 | 24 |
| 10 | Nature Communications | 2825 | United Kingdom | 16.6 | 5.116 | Q1 | 22 |
JCR, journal citation reports.
The journal’s dual-map overlay shows the relationship between citing and cited articles, with different colors indicating different citation relationships.17 The left side of the map can be seen as the application of the mRNA field, and the right side as the research foundation of the mRNA field. As shown in (Figure 6C), three main citation paths were identified: two green paths and one orange path. The green paths indicate that articles published in “Health, Nursing, Medicine” and “Molecular Biology, Genetics” journals are generally cited by articles in “Medicine, Medical, Clinical” journals. The orange path indicates that articles published in “Molecular Biology, Genetics” journals are generally cited by articles in “Molecular Biology, Immunology” journals.
Co-cited literature in the field of mRNA vaccines
We analyzed 1,679 co-cited documents, and the top 10 most-cited articles are listed in (Table 8). The article “Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine” published in the New England Journal of Medicine (158.5) ranked first, with 8,159 citations.18 Among these highly cited articles, research on mRNA vaccines against SARS-CoV-2 was most popular, especially regarding the vaccine-induced response and its safety and efficacy. Seven of the top ten articles were published in the New England Journal of Medicine, one of the most authoritative and cutting-edge journals in the medical field, with the remaining three in Nature and its sub-journals. Additionally, we used CiteSpace for co-citation network (Figure 6B). Given the profound impact of the COVID-19 pandemic on mRNA vaccine research, we have also summarized and analyzed the top 10 highly cited publications in this field, excluding COVID-19-related studies (Table 9). The top 10 highly cited non-COVID publications covered a wide range of years, from 2012 to 2020, with the majority published in high-impact journals such as Nature, Cell, and Nature Reviews Drug Discovery. These publications spanned various prestigious journals across multiple disciplines, including immunology, nanotechnology, molecular therapy, and genomic medicine, reflecting the interdisciplinary nature of mRNA vaccine research.
Table 8.
Top 10 highly cited publications in the field of mRNA vaccine.
| Rank | Title | Citation | Year | Journal | Type | Impact Factor (2022) |
|---|---|---|---|---|---|---|
| 1 | Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine | 8159 | 2020 | New England Journal of Medicine | Article | 158.5 |
| 2 | mRNA vaccines – a new era in vaccinology | 1949 | 2018 | Nature Reviews Drug Discovery | Review | 120.1 |
| 3 | An mRNA Vaccine against SARS-CoV-2-Preliminary Report | 1939 | 2020 | New England Journal of Medicine | Article | 158.5 |
| 4 | BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting | 1540 | 2021 | New England Journal of Medicine | Article | 158.5 |
| 5 | Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates | 1504 | 2020 | New England Journal of Medicine | Article | 158.5 |
| 6 | Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant | 1072 | 2022 | New England Journal of Medicine | Article | 158.5 |
| 7 | COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses | 1012 | 2020 | Nature | Article | 64.8 |
| 8 | Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults | 925 | 2020 | Nature | Article | 64.8 |
| 9 | Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults | 902 | 2020 | New England Journal of Medicine | Article | 158.5 |
| 10 | Covid-19 Breakthrough Infections in Vaccinated Health Care Workers | 838 | 2021 | New England Journal of Medicine | Article | 158.5 |
Table 9.
Top 10 highly cited Non-COVID publications in the field of mRNA vaccine.
| Rank | Title | Citation | Year | Journal | Type | Impact Factor (2022) |
|---|---|---|---|---|---|---|
| 1 | mRNA vaccines – a new era in vaccinology | 2235 | 2018 | Nature Reviews Drug Discovery | Review | 120.1 |
| 2 | Mutant MHC class II epitopes drive therapeutic immune responses to cancer | 835 | 2015 | Nature | Article | 64.8 |
| 3 | Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination | 584 | 2017 | Nature | Article | 64.8 |
| 4 | Modified mRNA Vaccines Protect against Zika Virus Infection | 551 | 2017 | Cell | Article | 64.5 |
| 5 | Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy | 465 | 2017 | Nano Letters | Article | 10.8 |
| 6 | An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma | 460 | 2020 | Nature | Article | 64.8 |
| 7 | Advances in the delivery of RNA therapeutics: from concept to clinical reality | 438 | 2017 | Genome Medicine | Review | 12.3 |
| 8 | Nonviral delivery of self-amplifying RNA vaccines | 429 | 2012 | Proceedings Of The National Academy Of Sciences Of The United States Of America | Article | 11.1 |
| 9 | Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses | 414 | 2017 | Molecular Therapy | Article | 12.4 |
| 10 | Advances in mRNA Vaccines for Infectious Diseases | 408 | 2019 | Frontiers In Immunology | Review | 7.3 |
During our literature collection process, we discovered that although some journals had relatively low annual publication volumes and overall article counts, they nevertheless maintained exceptional quality. For instance, Molecular Therapy (Q1, Impact Factor = 12.4) primarily focused on the applications and cutting-edge developments of mRNA vaccine technology in various infectious diseases and cancer treatments, such as multivalent and nucleoside-modified mRNA influenza vaccines, as well as clinical trials of mRNA vaccines against H10N8 and H7N9 influenza viruses,19–21 The research published in Molecular Therapy has made significant contributions to driving the progress of the mRNA vaccine field.
Keyword analysis in the field of mRNA vaccines
Keyword analysis helps describe the research status of a field and elucidate current research hotspots and future directions. We utilized VOSviewer and Citespace for visual and cluster analyses of keywords (Figure 7a,b). The top three keywords were “mRNA vaccine,” “immunogenicity,” and “covid 19,” appearing 682, 272, and 271 times, respectively, indicating that the development of anti-COVID-19 mRNA vaccines and the immune responses they elicit are the focal points of research in this area. Additionally, based on (Figure 7b), we can categorize the keyword clustering results into four domains:
Figure 7.
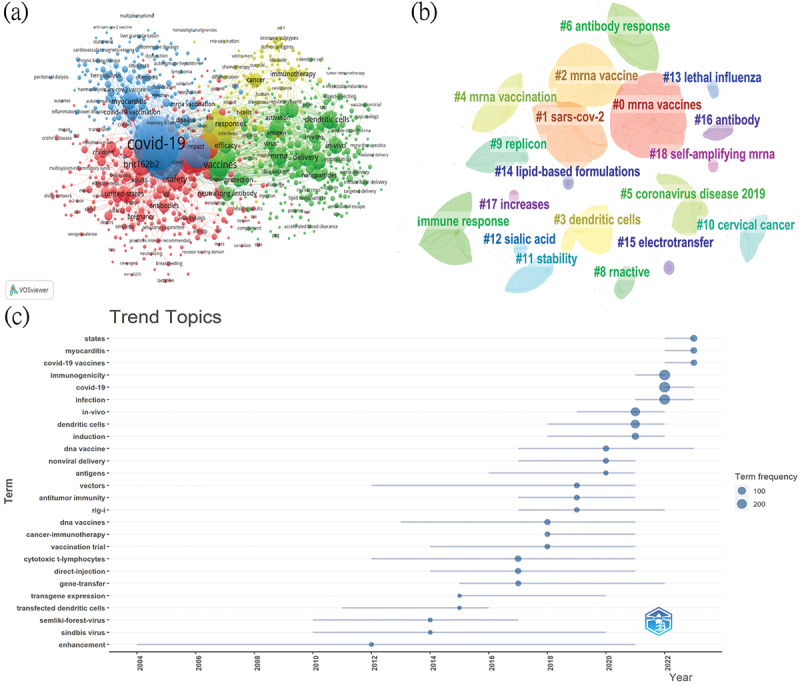
Network on keywords in the field of mRNA vaccines. (a) VOSviewer cluster visualization of keywords. (b) Cluster diagrams of keywords. (c) Visualization of keywords according to the average publication year (APY).
Research related to the application of mRNA vaccines in infectious diseases (#1 sars-cov-2, #5 coronavirus 2019, #13 lethal influenza, and blue cluster includes COVID-19 vaccination, disease, myocarditis).
Studies related to the body’s response to mRNA vaccines (#6 antibody response, #7 immune response, #16 antibody, with red cluster including antibodies, safety).
Research on mRNA vaccine delivery systems (#3 dendritic cells, #11 stability, #14 lipid-based formulations, with green cluster including dendritic cells, delivery).
Research on mRNA cancer vaccines (#10 cervical cancer, with yellow cluster including cancer, immunotherapy).
The thematic dynamics and research of keywords each year, as shown in (Figure 7C), indicate that from 2012 to 2015, enhancement, sindbis virus, Semliki-forest-virus, transgene expression, and transfected dendritic cells suggest that enhancing the gene transfer of viral mRNA in the body was a research hotspot, laying the foundation for future mRNA vaccine development. From 2016 to 2019, the topics of anti-tumor immunity, cancer immunotherapy, vectors, and rig-i indicate that the period’s hot topic was using mRNA vaccines to combat tumors, particularly studying their receptors and signaling pathways. From 2020 to 2023, keywords like antigens, COVID-19 vaccines, states, and myocarditis show that the recent research hotspots are the development of COVID-19 vaccines, focusing on how to improve the stability of mRNA vaccines and the side effects they may cause.
Discussion
During our literature search, in addition to literature related to mRNA vaccines, we also found a very small amount of literature on mRNA delivery of antibodies. mRNA delivery of antibodies is a new technology, involving the use of mRNA to code for antibodies or antibody-related proteins, and then delivering these mRNAs into human cells to induce the production of antibodies. This method may be helpful in treating certain diseases, especially those related to the immune system.22
General information
Based on the trend of annual publication output, the development of the field over the past two decades can be divided into two phases: an initial phase of very slow growth followed by a later phase of rapid increase. This change in the pace of development is likely due to the global pandemic of COVID-19, which spurred a large amount of research related to mRNA vaccines. As mRNA vaccines represent a novel vaccine technology, many scientists and research institutions have dedicated efforts to developing and improving mRNA vaccines in response to the pandemic. This has led to a surge in publications related to the development, efficacy, and safety of mRNA vaccines.23 However, the decline in the number of publications in 2023 might be attributed to a shift in research focus, saturation of research findings, and control of the COVID-19 pandemic.24 During this process, many key questions have been answered, and some core technologies have been improved and optimized, leading to a reduction in the number of publications.
Trends in collaboration networks among countries, institutions, and authors
There has been a long-standing imbalance in academic resources between developing and developed countries.25 For example, China, as the only developing country among the top ten, ranks second in the world in publication output but has less intermediacy centrality, weaker collaboration with other countries, and less influence on other nations. This phenomenon can be explained from two aspects: compared to developed countries, many scientific studies in developing countries tend to be repetitive and imitative. Secondly, there is a clear academic gap between most developed and developing countries. Developed countries have denser collaboration networks, and their intermediacy centrality is relatively higher. Most institutions with a high volume of publications are located in Europe and America. Interestingly, most of the top ten most-cited authors are from Pfizer, likely because the mRNA vaccine research by Kariko K and Weissman D in 2005 led to successful animal and human trials,26 with Pfizer/BioNTech and Moderna obtaining licenses for the technology developed at the University of Pennsylvania and using it for vaccine development.
Weissman D is the most prolific author in the field of mRNA vaccines, with 41 articles included in the WoS Core Collection, making him a highly influential and significant figure in driving the development of this field. Additionally, Nobel Prize winner Kariko K has the highest intermediacy centrality among all co-cited authors, proving her pivotal position in the field of mRNA vaccines. Her outputs are of high quality and widely referenced. The co-occurrence of institutions reveals that the University of Pennsylvania, the employer of the two laureates, also wields considerable influence in this field, producing 105 outputs over the past 20 years, ranking fifth among 400 institutions worldwide participating in this research. Its intermediacy centrality of 0.02, ACI of 75.05, and H-index of 36 among the top ten output institutions all rank third, proving that despite a high volume of output, its research maintains high quality and occupies a significant position in this field.
In the fields of base and nucleoside modifications and lipid nanoparticles, the teams led by the two Nobel laureates have closely collaborated with other organizations and scholars, objectively promoting the dissemination and exchange of this research, thereby advancing its development. Particularly in the field of lipid nanoparticles, as seen in the co-cited author diagram, Kariko K ranks first in intermediacy centrality and fourth in co-citations, highlighting her significant role in leading and advancing the application of lipid nanoparticle technology in mRNA vaccines. Her guidance has been instrumental in making lipid nanoparticles the most stable delivery system for mRNA vaccines.
Current research hotspots in the field of mRNA vaccines
Based on the current research landscape and considering the contributions and clustering of keywords, the main research hotspots in this field are the immune response to RNA vaccines, delivery systems, cancer applications, and infectious disease applications. Research on cancer and infectious diseases primarily targets clinical applications, immune response focuses more on basic immunological research, and the delivery system represents a multidisciplinary crossover study, such as medical-engineering and medical-chemistry intersections. These four hotspots are closely related; whether for tumor or infectious disease vaccines, often they serve merely as a “primer,” ultimately relying on inducing the body’s own immune system. Therefore, understanding the immune response to mRNA after entering the human body is fundamental for all clinical applications. The delivery system, as the “vehicle” for RNA, is responsible for stably transporting fragile RNA to target cells and releasing it, making an appropriate delivery system crucial for enhancing vaccine stability, target cell accuracy, and efficiency. Later in this document, we will discuss the current status and future prospects of these areas based on keywords, contributions, clusters, and emerging results.
Immune response to mRNA vaccines in the human body
mRNA vaccines are delivered to antigen-presenting cells, such as dendritic cells, macrophages, and B cells.27 Lipid nanoparticles (LNP) protect mRNA from extracellular ribonuclease degradation.28 Positively charged ionizable cationic lipids facilitate mRNA localization on negatively charged cell membranes, promoting endocytosis and subsequent endosomal escape.29 Post-translation, the related protein products are secreted from the host cell or processed into smaller peptides by the proteasome. Secreted protein products can directly serve as antigens to activate related immune responses. Processed antigen peptides are transported to the endoplasmic reticulum (not shown) and the Golgi apparatus and loaded onto major histocompatibility complex (MHC) class I or II molecules, subsequently presented on the cell surface. MHC I is recognized by cytotoxic T cells via the CD8+ receptor, and MHC II is recognized by helper T cells via the CD4+ receptor, leading to the production of inflammatory cytokines and subsequent induction of humoral and cell-mediated immune responses.30,31
An important area of research for mRNA vaccines is how to enable mRNA to evade the body’s immune system to prevent inflammatory responses that could attack the mRNA template. In 2005, scholars Drew Weissman and Katalin Karikó proposed that modifying certain nucleosides in RNA could significantly reduce the inflammatory response it triggers in the human body.32 Subsequent research has shown that certain modified nucleosides, like pseudouridine or its m1Ψ derivatives, replacing uridine in mRNA can greatly reduce the body’s recognition of mRNA and suppress the immune response it would trigger.32,33 For example, single-stranded RNA (ssRNA) and its degradation products primarily bind simultaneously to guanosine and the trinucleotide UUU on toll-like receptor (TLR) 7,34 and to uridine and the dinucleotide UG on TLR8.35 Modified pseudouridine nucleosides lack this binding capability. Also, modified nucleosides can inhibit the activation of various dsRNA sensors, including TLR3, retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), protein kinase R (PKR), and 2’−5’-oligoadenylate synthetase,36–38 Nucleoside modifications can also be performed through “capping,” typically using a cap structure (m7)GpppNm), characterized by methylation on the first cap-proximal nucleotide, disguising it as a self-substance in the human body.39,40 Another unexpected benefit of RNA modification is that it can increase the yield of mRNA expression products, thereby achieving a better immune effect.41 For example, an mRNA vaccine encoding the influenza virus HA only induced a strong Tfh cell, germinal center (GC), B cell, and antibody response in mice after m1Ψ modification, while no such response was observed without m1Ψ modification.42
mRNA vaccine delivery systems
For mRNA vaccines to exhibit their specific antigenic effects, they must enter the cytoplasm of cells, which is fundamental to vaccine efficacy.43 Studies indicate that only molecules smaller than 1000 Da can undergo passive transport, and most transport occurs at the cell membrane.44 Given the relatively large molecular weight of mRNA and its negative charge, and the fact that cell membranes, composed of phospholipid bilayers, also carry a negative charge, lipid carriers emerge as the most suitable form of delivery.45 Lipid carriers have a high encapsulation rate for mRNA molecules and exhibit strong targeting, affinity to body cells, and high biocompatibility. Some lipid carriers have shown the ability to deliver mRNA vaccines to target sites within the body in a noninvasive manner. Based on these characteristics, lipid carriers currently represent the best means of protecting mRNA molecules from enzymatic degradation.46,47
Further research involves encapsulating mRNA molecules into nanoparticles for intracellular delivery, namely lipid nanoparticles (LNPs), which are the preferred delivery system for current mRNA vaccines.48 Using LNPs as a carrier offers several other benefits, such as resistance to enzymatic degradation when administered intravenously.49 Furthermore, antibodies or nucleic acid aptamers targeting specific cell surface molecules can be incorporated into the mRNA-encapsulating nanoparticles, achieving targeted transport of mRNA to intended cells through antigen-antibody interaction or high-affinity binding of aptamers to receptors.48,50
In the therapeutic application of mRNA vaccines, tissue-targeted delivery of mRNA-based therapeutics is crucial for effective mRNA expression in the body.51 Currently, the most widely used LNP-based mRNA delivery is specifically targeted to the liver, with ongoing research focusing on LNP-based vaccine platforms for targeting other tissues.52 By adding selective organ-targeting molecules to LNPs and administering them intravenously, mRNA and Cas9 mRNA/sgRNA and Cas9 ribonucleoprotein complexes can be delivered to targeted tissues such as the liver, spleen, lungs, etc.53 Additionally, cell-targeted delivery of mRNA-based therapeutics (especially to antigen-presenting cells) plays a crucial role in controlling immune responses by transmitting necessary signals to T cells and activating their amplification and differentiation.54
Potential mRNA vaccine delivery systems also include peptide-based and polymer-based systems. Protamine, rich in arginine and belonging to cell-penetrating peptides (CPPs), is a rapidly developing peptide-based carrier.55 Although protamine can further stimulate the immune system as an adjuvant, existing clinical data shows that protamine-loaded mRNA vaccines offer low protective efficacy, possibly due to difficulties in endosomal escape.56 Polymer-based carriers, such as polyethyleneimine (PEI), are common cationic polymer carriers, but their large molecular structure brings certain toxicity.57 Some studies have shown that modifying PEI with fatty chains or esters can reduce its toxicity and improve delivery efficiency. Further research is needed to address these shortcomings.58
Application of mRNA vaccines in cancer
Based on cluster results #10 (cervical cancer), the evolution of keywords, and the emergence of cancer immunotherapy, mRNA cancer research has been a key direction in the field. mRNA cancer vaccines, based on mRNA technology, deliver mRNA molecules encoding specific antigens to the human body to trigger or enhance the immune system’s response against cancer cells, overcoming tumor immune escape caused by natural selection of tumor cell clones lacking immunogenic antigens. Currently, designers of mRNA cancer vaccines are enhancing T-cell responsiveness to relevant antigens by enhancing antigen immunogenicity, appropriately activating innate immunity, combining with adjuvants, and avoiding antibody-dependent enhancement (ADE)59 thereby strengthening the host’s resistance to viruses and tumors.
To date, mRNA cancer vaccines have shown potential and achieved encouraging results. For instance, NCT03323398 explores the application of mRNA vaccines in early-stage female breast cancer. In a study by Lina Liu and Yuhua Wang,60 an mRNA vaccine encoding the tumor antigen MUC1 was delivered to dendritic cells (DC) in lymph nodes via nanoparticles to activate and expand tumor-specific T cells. The study combined anti-CTLA-4 monoclonal antibodies with the mRNA vaccine, revealing that the mRNA vaccine successfully expressed tumor antigens in dendritic cells, significantly enhancing the in vivo anti-tumor immune response and successfully inhibiting the growth of breast cancer cells. These findings indicate some success in treating specific mutational antigens in patients with mRNA cancer vaccines. On December 14, 2023, Moderna, a U.S. pharmaceutical company, released the three-year follow-up results of its mRNA vaccine used in cancer treatment.61 In this Phase IIb clinical trial, known as Keynote-942, researchers assessed the efficacy of Moderna’s mRNA-4157 vaccine in conjunction with Merck’s Keytruda in patients with completely resected high-risk melanoma. The three-year follow-up data revealed that, compared to using Keytruda alone, the combination therapy significantly reduced the risk of recurrence or death by an impressive 49% in high-risk melanoma patients. Furthermore, concerning distant metastasis-free survival, the combination therapy demonstrated a remarkable 62% reduction in the risk of developing distant metastases or mortality compared to Keytruda alone.
Despite the great potential of mRNA cancer vaccines, challenges and obstacles remain in their application. Firstly, intrinsic factors of the molecule itself, such as the instability and degradability of mRNA, are major concerns, necessitating suitable carriers and delivery methods to ensure mRNA stability and efficiency.62 Secondly, individual differences and tumor heterogeneity pose barriers to vaccine development. Tumor heterogeneity, i.e., genomic heterogeneity of tumor cells leading to antigen heterogeneity, is a key factor affecting the generation of anti-tumor T cell responses.63 Therefore, identifying target antigens and developing personalized vaccine designs are challenging. Additionally, human leukocyte antigen (HLA) types and the tumor immune microenvironment profoundly impact the development of mRNA cancer vaccines.64,65 Given these factors, the heterogeneity of tumors and HLAs, as well as the development of neoantigen vaccines, have become hot topics in cancer vaccine research. Theoretically, neoantigen vaccines could offer more specific anti-tumor effects and weaker side effects compared to targeted vaccines against tumor-associated antigens. Considering the significant influence of immune cells, stromal cells, and various cytokines and tissue factors that constitute the tumor immune microenvironment, research on combined immunotherapy, such as using mRNA vaccines with immune adjuvants, has become a key trend in the field of cancer vaccine applications.66–68
The applications of mRNA vaccines in the field of infectious diseases
The current state of research in the field of mRNA vaccines, particularly in addressing infectious diseases, is evident through keyword clustering, including #1 SARS-CoV-2, #5 Coronavirus Disease 2019, and #13 Lethal Influenza. To date, numerous clinical trials are underway to explore the efficacy of mRNA vaccines in treating or preventing viral diseases such as coronaviruses, yellow fever, influenza viruses, respiratory syncytial virus, and more.69
During the recent COVID-19 pandemic caused by the novel coronavirus, mRNA vaccines played a pivotal role in disease prevention and outbreak control. Results from Phase III clinical trials indicate that both BNT162b2 and mRNA-1237 vaccines exhibit high effectiveness. While there is a slight fluctuation in efficacy across age groups, both vaccines demonstrate acceptable protective responses in all age brackets (BNT162b2 with a preventive rate of 94% for individuals aged 65 and above; mRNA-1237 with vaccine efficacy rates of 95.6% for individuals aged 18–65 and 86.4% for those aged 65 and above.70
In real-world scenarios with widespread vaccine administration, BNT162b2 and mRNA-1237 vaccines continue to display outstanding effectiveness (mRNA-1273 efficacy at 96.2%, BNT162b2 at 98.2%.70 Regarding safety, adverse reaction data voluntarily reported by 3.644 million individuals vaccinated with mRNA coronavirus vaccines between December 14, 2020, and February 28, 2021, collected by the Centers for Disease Control and Prevention (CDC) and published in the Journal of the American Medical Association (JAMA), suggest that adverse reactions are generally minor and mostly short-term,71–73 This underscores the critical role of mRNA vaccines in infectious disease prevention. Recent research has extended the application of mRNA vaccines to the herpes zoster virus (VZV).74 This study showcased a novel mRNA vaccine called ZOSAL and its experimental results in mice and rhesus macaques. These experimental results suggest that the ZOSAL mRNA platform holds advantages for VZV vaccine development and may serve as a potential direction for next-generation vaccine development.
Studies suggest a correlation between the strength of the immune response and the likelihood of adverse reactions, indicating that individuals with robust immune responses may experience more reactions post-vaccination. Moreover, mRNA vaccines have been applied to combat common viral infections. Both Moderna’s quadrivalent seasonal influenza candidate vaccine and Pfizer’s quadrivalent mRNA influenza vaccine demonstrated positive outcomes in Phase III studies, showing immune responses against four viral strains.
mRNA vaccines also exhibit significant potential in combating bacterial diseases. In a recent study, Pavot et al.75 developed a novel Lyme disease vaccine using mRNA technology, effectively eliciting an immune response for treating or preventing Lyme disease while maintaining excellent stability.
Despite extensive preclinical and clinical studies demonstrating the efficacy of mRNA vaccines against viral and bacterial diseases, research on parasitic diseases remains limited. Duthie et al. .76 have developed a naked mRNA replicon encoding the LEISH-F2 gene to combat Leishmania parasite infection. Results indicate a significant reduction in liver parasite burden when using a stable water-in-oil emulsion (SLA-SE) as a carrier for F2-RNA vaccine administration, demonstrating the enhanced ability of recombinant LEISH-F2 protein to boost resistance to parasitic infection.
Additionally, mRNA vaccines show promise in combating Toxoplasma infections. In Luo et al.60’s study, injecting a homemade PREP-NTPase-II-type vaccine into mice increased survival time and rates compared to the control group, proving the ability of mRNA vaccines to enhance mouse resistance to Toxoplasma.
Although research on the efficacy of mRNA vaccines against parasitic infections is limited, the cost-effectiveness of mRNA vaccines, coupled with the lack of vaccines for parasitic diseases, suggests that the mRNA vaccine platform may be well-suited for developing overlooked parasitic vaccines.77
In summary, mRNA vaccines demonstrate significant potential in preventing infectious diseases. The global impact of the 2019 COVID-19 pandemic caused by the SARS-CoV-2 virus highlights the substantial progress in mRNA vaccine development.72 Throughout the pandemic response, mRNA vaccines played a crucial role,78–80 showcasing their potential in the field of infectious diseases. While various mRNA vaccines still possess distinct drawbacks, such as the potential for unnecessary immune reactions in self-amplifying mRNA and occasional inadequacy of traditional mRNA vaccines in eliciting desired immune responses, ongoing research and development in this emerging field are likely to overcome these challenges in the future.
Adverse effects and safety considerations
While mRNA vaccines have demonstrated remarkable efficacy, it is crucial to address the potential adverse effects and safety concerns associated with their use, particularly those reported during the COVID-19 pandemic. A range of mild adverse reactions, such as injection site pain, fatigue, headache, fever, chills, and muscle aches, have been widely observed following mRNA vaccine administration.81 These reactions are typical of vaccine administration and generally do not interfere with daily activities. Although severe adverse events have been recorded, these occurrences are extremely rare within the overall vaccinated population. Severe events include allergic reactions, cardiovascular events, and neurological reactions.82 Despite the occurrence of adverse reactions, most vaccines have expressed openness to receiving the second dose, indicating a high acceptance and trust in the vaccines.81 Research has also revealed a diversity in the symptoms and severity of adverse reactions, ranging from mild local reactions to more severe systemic reactions.81,83 Interestingly, some studies have suggested that the gut microbiome composition of vaccines may be associated with vaccine immunogenicity and adverse events, indicating that certain probiotics could potentially reduce the risk of adverse reactions by modulating the immune response.84 These findings aid healthcare professionals in better understanding the safety profile of mRNA vaccines and considering strategies to mitigate adverse reactions, thereby improving vaccine acceptance and coverage rates. Simultaneously, they emphasize the importance of continuous monitoring and analysis of vaccine adverse events to ensure the highest levels of safety and efficacy.
Future trends in mRNA vaccine development
The success of mRNA technology in the development and application of COVID-19 vaccines has paved the way for new possibilities in the future of the medical field. The maturity of various technologies and the extensive use of nanotechnology in developing mRNA vaccine delivery carriers have significantly propelled research in the mRNA vaccine domain. The triumph of COVID-19 vaccines also suggests the feasibility of utilizing mRNA vaccines for both disease prevention and treatment.
The research directions and potential applications in the field of mRNA vaccines are vast, and future development trends are speculated to encompass the following aspects:
Vaccine Improvement and Optimization: Despite the notable success of mRNA vaccines during the COVID-19 pandemic, there are still numerous avenues for optimization. Areas such as enhancing vaccine stability, reducing transportation and storage challenges, exploring optimal injection methods, and refining administration protocols offer opportunities for improvement.
Research and Preparation of Personalized Vaccines: With the rise of personalized medicine, the investigation of personalized treatment plans using mRNA vaccines is one of the research directions. This involves designing vaccines tailored to specific diseases based on individual genomic, immunological characteristics, and other physiological parameters.
Applications in Cardiovascular Diseases: Studies suggest that mRNA, when delivered without causing inflammatory reactions, can induce antigen-specific immune tolerance.85 This discovery provides a new perspective on applying mRNA vaccines to atherosclerosis.86
Research in Cancer Treatment: Cancer is a major global health concern, and mRNA technology holds immense potential in cancer vaccines. Currently, mRNA cancer vaccines are undergoing Phase III clinical trials. Future research in the mRNA vaccine field will focus on enhancing the immune system’s ability to attack specific tumors and overcoming the inhibitory effects of the tumor immune microenvironment in the development of specific cancer vaccines.
Conclusions
In summary, the 2023 Nobel Prize in Biomedical Sciences was awarded to two scientists from the University of Pennsylvania, and the application of bibliometric analysis has better showcased their significant contributions in the field, revealing the progress made in mRNA vaccine research over the past 20 years. The research on mRNA vaccines still holds great prospects. Despite the immense academic influence of North America and Europe, institutions from Japan and some developing countries, led by China, have shown boundless potential in this field. There remain uncertainties in the specific immune response processes of mRNA vaccines within the human body, how to better maintain the stability of their delivery process, their application in the treatment and prevention of cancer, and the prevention of more infectious diseases. These clinical challenges have not only garnered attention in recent years but have also become focal points for future research.
Funding Statement
The funding for this work was the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0148), Beijing Postdoctoral Research Funding Project (2023-ZZ-002) and China Postdoctoral Science Foundation funded project (2023M742438).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Author Chaobin Zhang and Yuhang Wang did the experiments, analyzed data, and wrote the manuscript. Author Jianding Peng, Xiaotian Wen, Youwen Zhang and Wanbo Tai analyzed data and revised the manuscript. Author Kejun Li, Hanjian Du and Xiaofei Hu supervised this project. All authors have read the final manuscript and approved it for publication.
Availability of data and materials
The data that support the findings of this study are available from the corresponding public databases and academic journals. These data were derived from the following resources: [https://webofscience.help.clarivate.com/Content/wos-core-collection/wos-core-collection.htm]. Further details about the datasets, including how to access them, are available from the authors upon reasonable request. No new data were generated or analyzed in this study.
Consent for publication
All data and materials used in this research are publicly available, de-identified, or collected in a manner that does not require consent for publication. There are no details, images, or videos relating to individual participants. As such, consent for publication is not applicable for this study.
Ethical approval and consent to participate
This study did not involve human or animal subjects, and therefore did not require formal ethical approval. All research activities were conducted in accordance with ethical standards for research without direct human or animal participation.
References
- 1.Peck M, Gacic-Dobo M, Diallo MS, Nedelec Y, Sodha SS, Wallace AS.. Global routine vaccination coverage. MMWR Morb Mortal Wkly Rep. 2018;68(42):937–18. doi: 10.15585/mmwr.mm6842a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghattas M, Dwivedi G, Lavertu M, Alameh MG. Vaccine technologies and platforms for infectious diseases: current progress, challenges, and opportunities. Vaccines. 2021;9(12):1490. doi: 10.3390/vaccines9121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S, Yang K, Li R, Zhang L. mRNA vaccine era–mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21(18):6582. doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390(10101):1511–20. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 5.Granot-Matok Y, Ezra A, Ramishetti S, Sharma P, Naidu GS, Benhar I, Peer D. Lipid nanoparticles-loaded with toxin mRNA represents a new strategy for the treatment of solid tumors. THERANOSTICS. 2023;13(11):3497–508. doi: 10.7150/thno.82228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Miguel AG, Gil-Prieto R. Vaccination strategies against SARS-CoV-2: General impact on the development of the pandemic. Revista Espanola De Quimioterapia. 2021;34(Suppl 1):60–2. doi: 10.37201/req/s01.18.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guler AT, Waaijer CJ, Palmblad M. Scientific workflows for bibliometrics. Scientometrics. 2016;107(2):385–98. doi: 10.1007/s11192-016-1885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radu AF, Bungau SG, Negru PA, Marcu MF, Andronie-Cioara FL. In-depth bibliometric analysis and current scientific mapping research in the context of rheumatoid arthritis pharmacotherapy. Biomed Pharmacother. 2022;154:113614. doi: 10.1016/j.biopha.2022.113614. [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study. Front Oncol. 2020;10:627891. doi: 10.3389/fonc.2020.627891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608. doi: 10.1517/14712598.2012.674507. [DOI] [PubMed] [Google Scholar]
- 11.Zheng B, Kuang Y, Yuan D, Huang H, Liu S. The research landscape of immunology research in spinal cord injury from 2012 to 2022. Jor Spine. 2023;6(3):e1261. doi: 10.1002/jsp2.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Zhao H, Fu L, Cui J, Yang Y. Global trends and research progress of photodynamic therapy in skin cancer: a bibliometric analysis and literature review. Clin Cosmet Investig Dermatol. 2023;16:479–98. doi: 10.2147/CCID.S401206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J. The global research of artificial intelligence on prostate cancer: a 22-year bibliometric analysis. Front Oncol. 2022;12:843735. doi: 10.3389/fonc.2022.843735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami GG, Labib T. Modeling COVID-19 transmission dynamics: a bibliometric review. Int J Environl Res Public Health. 2022;19(21):14143. doi: 10.3390/ijerph192114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh KE, Flaherty GT. Travel medicine research in the new millennium: A bibliometric analysis of articles published in travel medicine and infectious disease, 2003-2019. Travel Med Infect Dis. 2020;33:101549. doi: 10.1016/j.tmaid.2019.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–75. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 17.Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J Assoc Inf Sci Technol. 2014;65(2):334–51. doi: 10.1002/asi.22968. [DOI] [Google Scholar]
- 18.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA covid-19vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, Tam YK, Madden TD, de Souza Ferreira LC, Weissman D. et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol Ther. 2020;28(7):1569–84. doi: 10.1016/j.ymthe.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against h10n8 and h7n9 influenza viruses. Mol Ther. 2017;25(6):1316–27. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H. et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower Doses. Mol Ther. 2018;26(2):446–55. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. NATURE. 2012;481(7379):81–U88. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S. et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–31. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nane GF, Robinson-Garcia N, van Schalkwyk F, Torres-Salinas D. COVID-19 and the scientific publishing system: growth, open access and scientific fields. Scientometrics. 2023;128(1):345–62. doi: 10.1007/s11192-022-04536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Wende M. International academic mobility: Towards a concentration of the minds in europe. Eur Rev. 2015;23(S1):70–88. doi: 10.1017/s1062798714000799. [DOI] [Google Scholar]
- 26.Aygün I, Barciszewski J. The forerunners and successful partnerships behind the BioNTech mRNA vaccine. J Appl Genet. 2023;65(1):47–55. doi: 10.1007/s13353-023-00793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, Xiao P, Araínga M, Shirreff LM, Pitard B. et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat Biomed Eng. 2019;3(5):371–80. doi: 10.1038/s41551-019-0378-3. [DOI] [PubMed] [Google Scholar]
- 28.Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdisciplinary Reviews-Nanomedicine And Nanobiotechnology. 2017;9(5). doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- 29.Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res. 2022;55(1):2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 30.Salleh MZ, Norazmi MN, Deris ZZ. Immunogenicity mechanism of mRNA vaccines and their limitations in promoting adaptive protection against SARS-CoV-2. PeerJ. 2022;10:e13083. doi: 10.7717/peerj.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang F, Lindgren G, Lin A, Thompson EA, Ols S, Röhss J, John S, Hassett K, Yuzhakov O, Bahl K. et al. Efficient Targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25(12):2635–47. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological Stability. Mol Ther. 2008;16(11):1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang ZK, Ohto U, Shibata T, Krayukhina E, Taoka M, Yamauchi Y, Tanji H, Isobe T, Uchiyama S, Miyake K. et al. Structural analysis reveals that toll-like receptor 7 is a dual receptor for guanosine and single-stranded rNA. Immunity. 2016;45(4):737–48. doi: 10.1016/j.immuni.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, Miyake K, Shimizu T. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nature Structural & Molecular Biology. 2015;22(2):109–15. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 36.Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, Karikó K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010;38(17):5884–92. doi: 10.1093/nar/gkq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson BR, Muramatsu H, Jha BK, Silverman RH, Weissman D, Kariko K. Nucleoside modifications in RNA limit activation of 2-5-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39(21):9329–38. doi: 10.1093/nar/gkr586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu X, Greenwald E, Ahmad S, Hur S. An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 2018;46(10):5239–49. doi: 10.1093/nar/gky177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardi N, Muramatsu H, Weissman D, Kariko K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 40.Henderson JM, Ujita A, Hill E, Yousif‐Rosales S, Smith C, Ko N, McReynolds T, Cabral CR, Escamilla‐Powers JR, Houston ME. et al. Cap 1 messenger RNA synthesis with Co-transcriptional CleanCap® Analog by in vitro transcription. Curr Protocol. 2021;1(2):e39. doi: 10.1002/cpz1.39. [DOI] [PubMed] [Google Scholar]
- 41.Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–88. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD. et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA. 2016;113(43):6639–48. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Ma X, Yue Y, Zhang K, Cheng K, Feng Q, Ma N, Liang J, Zhang T, Zhang L. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv Mater. 2022;34(20). doi: 10.1002/adma.202109984. [DOI] [PubMed] [Google Scholar]
- 44.Niazi SK. Making COVID-19 mRNA vaccines accessible: challenges resolved. Expert Rev Vaccines. 2022;21(9):1163–76. doi: 10.1080/14760584.2022.2089121. [DOI] [PubMed] [Google Scholar]
- 45.Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi: 10.1016/j.addr.2022.114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Zhang Z, Wang L, Chen K, Wang Y. mRNA vaccine delivery carrier useful for preparing mRNA vaccine and medicine for preventing and/or treating tumors, viral pneumonia or related diseases, is prepared using polyethylene glycol-polyphosphate block copolymer and cationic lipid. CN116763933-A.
- 47.Xia X, Zhang H, Wu J, You X. mRNA vaccine delivery vector, is lipid polymer, obtained by reacting polyamidoamine dendrimer with 1,2-epoxydodecane. CN110448695-A; CN110448695-B.
- 48.Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles–From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. Acs Nano. 2021;15(11):16982–7015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 49.Ji AF, Xu M, Pan Y, Diao L, Ma L, Qian L, Cheng J, Liu M. Lipid microparticles show similar efficacy with lipid nanoparticles in delivering mRNA and preventing cancer. Pharm Res. 2023;40(1):265–79. doi: 10.1007/s11095-022-03445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldosari BN, Alfagih IM, Almurshedi AS. Lipid Nanoparticles as delivery systems for RNA-Based vaccines. Pharmaceutics. 2021;13(2):206. doi: 10.3390/pharmaceutics13020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–28. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shobaki N, Sato Y, Harashima H. Mixing lipids to manipulate the ionization status of lipid nanoparticles for specific tissue targeting. Int J Nanomed. 2018;13:8395–410. doi: 10.2147/IJN.S188016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol. 2020;15(4):313–20. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guevara ML, Persano S, Persano F. Lipid-Based vectors for therapeutic mRNA-Based anti-cancer vaccines. Curr Pharm Des. 2019;25(13):1443–54. doi: 10.2174/1381612825666190619150221. [DOI] [PubMed] [Google Scholar]
- 55.Arangoa MA, Düzgünes N, de Ilarduya CT. Increased receptor-mediated gene delivery to the liver by protamine-enhanced-asialofetuin-lipoplexes. Gene Ther. 2003;10(1):5–14. doi: 10.1038/sj.gt.3301840. [DOI] [PubMed] [Google Scholar]
- 56.Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, Corral G, De Block EJL, Ecker L, Gabor JJ. et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329–40. doi: 10.1016/s1473-3099(21)00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–64. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren J, Cao Y, Li L, Wang X, Lu H, Yang J, Wang S. Self-assembled polymeric micelle as a novel mRNA delivery carrier. J Controlled Release. 2021;338:537–47. doi: 10.1016/j.jconrel.2021.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. NATURE. 2023;618(7963):144–50. doi: 10.1038/s41586-023-06063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo F, Zheng L, Hu Y, Liu S, Wang Y, Xiong Z, Hu X, Tan F. Induction of Protective Immunity against Toxoplasma gondii in Mice by Nucleoside Triphosphate Hydrolase-II (NTPase-II) self-amplifying RNA Vaccine Encapsulated in Lipid Nanoparticle (LNP). Front Microbiol. 2017;8:605. doi: 10.3389/fmicb.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.https://investors.modernatx.com/news, 2023).
- 62.He Q, Gao H, Tan D, Zhang H, Wang J. Z. mRNA cancer vaccines: Advances, trends and challenges. Acta Pharm Sin B. 2022;12(7):2969–89. doi: 10.1016/j.apsb.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast Cancer. Mol Ther. 2018;26(1):45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krishna S, Anderson KS. T-Cell epitope discovery for therapeutic cancer vaccines. Methods Mol Biol. 2016;1403:779–96. doi: 10.1007/978-1-4939-3387-7_45. [DOI] [PubMed] [Google Scholar]
- 65.Kreiter S, Diken M, Selmi A, Diekmann J, Attig S, Hüsemann Y, Koslowski M, Huber C, Türeci Ö, Sahin U. et al. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 2011;71(19):6132–42. doi: 10.1158/0008-5472.CAN-11-0291. [DOI] [PubMed] [Google Scholar]
- 66.Li JY, Wu Y, Xiang J, Wang H, Zhuang Q, Wei T, Cao Z, Gu Q, Liu Z, Peng R. et al. Fluoroalkane modified cationic polymers for personalized mRNA cancer vaccines. Chem Eng J. 2023;456:456. doi: 10.1016/j.cej.2022.140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bafaloukos D, Gazouli I, Koutserimpas C, Samonis G. Evolution and progress of mRNA vaccines in the treatment of melanoma: Future prospects. Nato Adv Sci Inst Se. 2023;11(3):636. doi: 10.3390/vaccines11030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, Ding J, Chen Y, Aduluso D, Zetter BR. et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. BIOMATERIALS. 2021;266:120431. doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou W, Jiang L, Liao S, Wu F, Yang G, Hou L, Liu L, Pan X, Jia W, Zhang Y. et al. Vaccines’ new Era-RNA Vaccine. Viruses. 2023;15(8):1760. doi: 10.3390/v15081760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Shi X, He W, Zhong G, Tang Y, Wang H, Zhang P. mRNA vaccine-a desirable therapeutic strategy for surmounting COVID-19 pandemic. Hum Vaccines Immunother. 2022;18(1):2040330. doi: 10.1080/21645515.2022.2040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rice SM, Ferree SD, Mesinkovska NA, Kourosh AS. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. International Journal of Women’s Dermatology. 2021;7(2):209–12. doi: 10.1016/j.ijwd.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–1. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang L, Zhao T, Zhao W, Shao A, Zhao H, Ma W, Gong Y, Zeng X, Weng C, Bu L. et al. Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques. Emerging Microbes Infect. 2024;13:2309985. doi: 10.1080/22221751.2024.2309985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kung F, Kaur S, Smith AA, Yang X, Wilder CN, Sharma K, Buyuktanir O, Pal U. A Borrelia burgdorferi surface-exposed transmembrane protein lacking detectable immune responses supports pathogen persistence and constitutes a vaccine target. J Infect Dis. 2016;213(11):1786–95. doi: 10.1093/infdis/jiw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duthie MS, Van Hoeven N, MacMillen Z, Picone A, Mohamath R, Erasmus J, Hsu F-C, Stinchcomb DT, Reed SG. Heterologous immunization with defined RNA and subunit vaccines enhances T cell responses that protect against leishmania donovani. Front Immunol. 2018;9:2420. doi: 10.3389/fimmu.2018.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Versteeg L, Almutairi MM, Hotez PJ, Pollet J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines. 2019;7(4):122. doi: 10.3390/vaccines7040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, Scearce R, Barr M, Eaton A, Hernandez G. et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines. 2021;6(1). doi: 10.1038/s41541-021-00307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paloncyová M, Cechová P, Srejber M, Kührová P, Otyepka M. Role of ionizable lipids in SARS-CoV-2 vaccines as revealed by molecular dynamics simulations: from membrane structure to interaction with mRNA fragments. J Phys Chem Lett. 2021;12(45):11199–205. doi: 10.1021/acs.jpclett.1c03109. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhary N, Weissman D, Whitehead K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20(11):817–38. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–81. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fraiman J, Erviti J, Jones M, Greenland S, Whelan P, Kaplan RM, Doshi P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine. 2022;40(40):5798–805. doi: 10.1016/j.vaccine.2022.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganesan S, Al Ketbi LMB, Al Kaabi N, Al Mansoori M, Al Maskari NN, Al Shamsi MS, Alderei AS, El Eissaee HN, Al Ketbi RM, Al Shamsi NS. et al. Vaccine side effects following COVID-19 vaccination among the residents of the UAE—An observational study. Front Public Health. 2022;10:876336. doi: 10.3389/fpubh.2022.876336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ng SC, Peng Y, Zhang L, Mok CK, Zhao S, Li A, Ching JY, Liu Y, Yan S, Chan DLS. et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71(6):1106–16. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371(6525):145–53. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 86.Furlan R. A Tolerizing mRNA Vaccine against Autoimmunity? Mol Ther. 2021;29(3):896–7. doi: 10.1016/j.ymthe.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding public databases and academic journals. These data were derived from the following resources: [https://webofscience.help.clarivate.com/Content/wos-core-collection/wos-core-collection.htm]. Further details about the datasets, including how to access them, are available from the authors upon reasonable request. No new data were generated or analyzed in this study.


