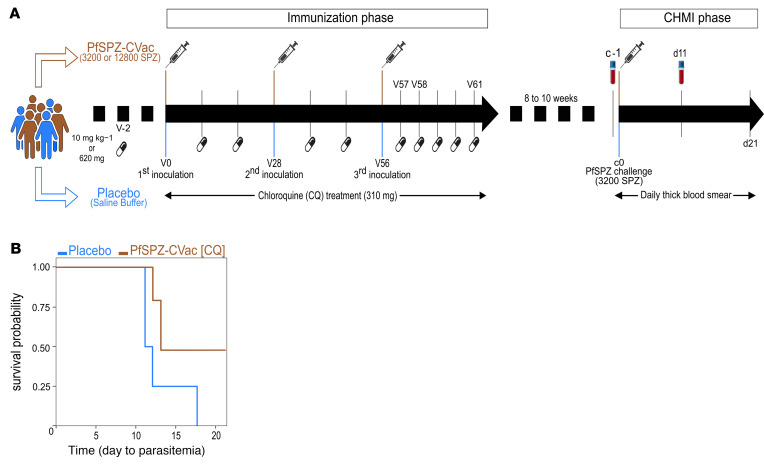
Figure 1. TüCHMI trial and outcome.
(A) Healthy volunteers included in the trial were split in 2 main groups: the experimental group (in brown) consisted of volunteers (n = 8) receiving 3 doses of PfSPZ vaccine at 28-days intervals (V0, V28, and V56) in combination with a weekly dose of chloroquine up to 5 days after the last inoculation (V61) (PfSPZ-CVac [CQ]), and the placebo group (in blue), which consisted of volunteers (n = 4) inoculated with saline buffer. Eight to 10 weeks after the last inoculation, all volunteers in both the experimental and placebo groups underwent a CHMI trial. Immune responses to PfSPZ-CVac [CQ] inoculation were assessed at c–1 (1 day before the challenge [c0]) and d11 (11 days after the challenge). (B) Proportion of protected volunteers. Kaplan-Meier survival curves for days to parasitemia determined by thick blood smear for PfSPZ-CVac [CQ]–vaccinated (brown) and placebo (blue) groups. Volunteers in the placebo group all became malaria positive by day 18 after CHMI, while in the vaccinated group, some volunteers (4 of 8 volunteers vaccinated in total) remained malaria negative up to 21 days after CHMI.