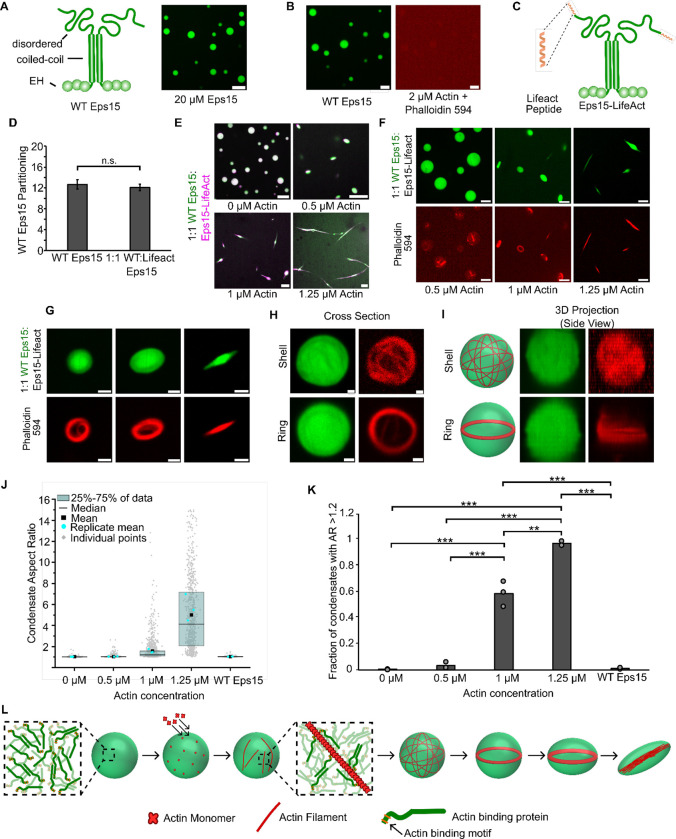
Figure 6. Adding an actin-binding domain to an arbitrary condensate-forming protein is sufficient to confer the ability to polymerize and bundle actin filaments.
A) Left: Schematic depicting wild type Eps15 and its major domains. Right: 20 μM wild type Eps15 forms condensates in solution with 3% (w/v) PEG. Scale bar 5 μm. B) Wild-type Eps15 (green) condensates do not polymerize or bundle actin as indicated by the lack of condensate deformation and a lack of phalloidin-stained filaments. Scale bars 5 μm. C) Schematic depicting fusion of the Lifeact peptide to the C-terminus of Eps15. D) Partitioning of wild type Eps15 in condensates formed from only wild type Eps15 (self-partitioning) and condensates formed with 1:1 wild type Eps15 and Eps15-Lifeact. Six fields of view were analyzed for each condition with more than 6 condensates analyzed for partitioning per field of view. Error bars indicate standard deviation. E) Representative images of condensate deformation and actin polymerization upon the addition of increasing concentrations of monomeric actin to condensates formed from wild-type Eps15 and Eps15-Lifeact. Scale bars 5 μm. F) Representative images of phalloidin staining of condensates consisting of wild-type Eps15 and Eps15-Lifeact following actin addition. Scale bars 5 μm. G) Representative confocal cross-section images of the progressive, actin-driven deformation of condensates consisting of wild-type Eps15 and Eps15-Lifeact. Scale bars 2 μm. H) Representative 2D confocal images of independent 1:1 WT Eps15:Eps15-Lifeact condensates (green) containing peripheral actin, shown with phalloidin staining (red), in both a shell of actin (top) and a ring of actin (bottom). Scale bars 1μm. I) 3D reconstructions of the same condensates shown in H demonstrating a shell of actin (top) and a ring of actin (bottom). J) Distribution of condensate aspect ratios across the conditions in E, with at least 700 condensates analyzed for each condition. For the 1.25 μM actin condition, values for aspect ratios higher than 15, corresponding to 3.5% of the data, are not displayed to better visualize distributions for all conditions. The mean is the mean of all data points in each condition, while the replicate mean is the mean of each of the individual replicates for each condition. K) Quantification of the fraction of elongated protein condensates, defined as condensates with aspect ratios > 1.2, across the conditions in E. Data are mean +/− standard deviation across three independent experiments with at least 700 condensates analyzed in total per condition. Overlayed gray circles denote the means of each replicate. Two asterisks denote p<.01, and three asterisks denote p<.001 using an unpaired, two-tailed t-test on the means of the replicates N=3. L) Cartoon depicting the proposed mechanism of condensate-driven actin polymerization and bundling by condensates of actin-binding proteins. All experiments were performed in a buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 5 mM TCEP, and 3% (w/v) PEG 8000.