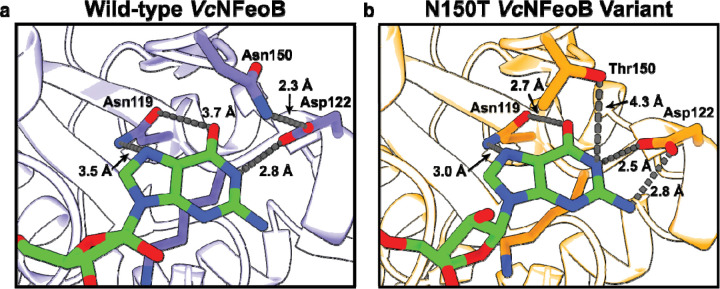
Fig. 4.
Comparisons of the nucleotide-binding pockets of the WT (a) and N150T (b) VcNFeoB NTPase domain structures in their GDP-bound forms. Fewer hydrogen-bonding interactions are observed in the WT GDP-bound structure compared to the N150T variant of the NTPase domain. We hypothesize that the fewer hydrogen-bonding interactions in the WT structure allow for greater plasticity in NTP/NDP binding, unlike the strict GTPases that do not bind and hydrolyze other NTPs.