Abstract
Mysids, besides krill, play a significant role in energy transfer and carbon sequestration. The ecology of coastal species is better understood than that of deep dwelling species such as Boreomysis arctica. The objectives of this study were to quantify spatiotemporal variations in body condition and the trophic level of B. arctica in autumn and winter, under sea-ice conditions in the St. Lawrence system, using a multimarker approach. We sampled along a 1000 km transect. Mean abundances in winter were higher in the estuary compared to the Gulf of St. Lawrence. Body condition, measured as total lipid content, was higher in winter than in autumn. Lipids of B. arctica were mainly composed of wax esters, thereby B. arctica is richer in energetic lipids compared to the three dominant krill species. We also observed seasonal differences in the trophic level of B. arctica, revealing carnivorous behavior in autumn compared to omnivory in winter. High intra-specific variability in both energetic strategy and feeding behavior was found that is potentially due to opportunistic feeding. Energy rich reserves suggest that B. arctica could act as a valuable prey for both benthic and pelagic consumers and thus playing a key role in bentho-pelagic energy transfer.
Keywords: ecophysiological condition, lipids, trophic ecology, Boreomysis arctica, St. Lawrence
INTRODUCTION
Mysids are a major component of estuarine and coastal zooplankton communities due to the high abundance, biomass and widespread distribution. They occupy an important link in marine food webs by holding a key position between benthos, plankton and nekton (Tattersall and Tattersall, 1951; Mauchline, 1980; Fulton, 1982). About 780 species within 120 genera of Mysidacea are known (Mauchline, 1980). Most of the species are marine and predominantly shallow water organisms, living in close vicinity to the sediment surface. The ecology of neritic mysids is relatively well documented, whereas ecological information for deep-dwelling species is very scarce. One of these species, B. arctica, has a wide distribution throughout the Northern Hemisphere (Mauchline, 1980; Brunel et al., 1998), mostly in the North Atlantic and the Mediterranean Sea (Macquart-Moulin, 1993; Cartes and Sorbe, 1998), occupying meso- and bathypelagic habitats down to 1400 m. In the Estuary and the Gulf St. Lawrence (EGSL), B. arctica is found in areas of depths between 200 and 500 m. It was occasionally observed to explore the water column at 150 m, but was never found in surface waters (0–50 m; Harvey et al., 2009). In the Lower St. Lawrence Estuary (LSLE) and the north-western GSL (Fig. 1), B. arctica was consistently found in samples from the deep channel habitats with high mean abundances up to 125 ind.m−2 (Descroix et al., 2005). These abundances suggest that B. arctica is an important contributor to the macrozooplankton community besides the three dominant krill species, Meganycthiphanes novegica, Thysanoessa raschii and T. inermis also occurring in very high abundance in the St. Lawrence ecosystem. While the distribution and population dynamics (e.g. Simard et al., 1986; Simard and Lavoie, 1999; Maps et al., 2014, 2015; Plourde et al., 2016; Benkort et al., 2019) and more recently the ecology (Plourde et al., 2011; Ollier et al., 2018; Cabrol et al., 2019a, b, 2020; Cope et al., 2021) of the three krill species are well documented, the ecology of B. arctica, especially in the EGSL, is mostly unknown. In contrast to the three krill species, no diel vertical migration pattern to food rich surface layers has been observed (Harvey et al., 2009). As a consequence, B. arctica in the St. Lawrence system will experience relatively constant physicochemical conditions during its entire life cycle (Galbraith et al., 2020) without direct access to freshly produced phytoplankton biomass in the photic zone. Therefore, B. arctica seems to be dependent on the organic matter flow from the surface layer into the deep water and/or on the zooplankton residing in these deep zones to acquire food. Even, if B. arctica lives <150 m under relatively stable temperature and salinity conditions year-round, the upper part of the water column exhibits high seasonal contrasts, including ice-cover, negative water temperatures and low light regime in winter reducing phytoplankton productivity compared with summer temperatures and good light conditions throughout spring to autumn (Galbraith et al., 2020; Blais et al., 2021). Furthermore, local upwelling in the LSLE induces a prolonged phytoplankton production, resulting in chlorophyll a concentration of > 1 mg.m−3 from May to October (Plourde et al., 2011; Blais et al., 2021). In contrast, the phytoplankton productivity in the Gulf of St. Lawrence is more patchy in space and time (Blais et al., 2021), strongly affecting local carbon export to the bottom and consequently food availability and quality to deep-dwelling species.
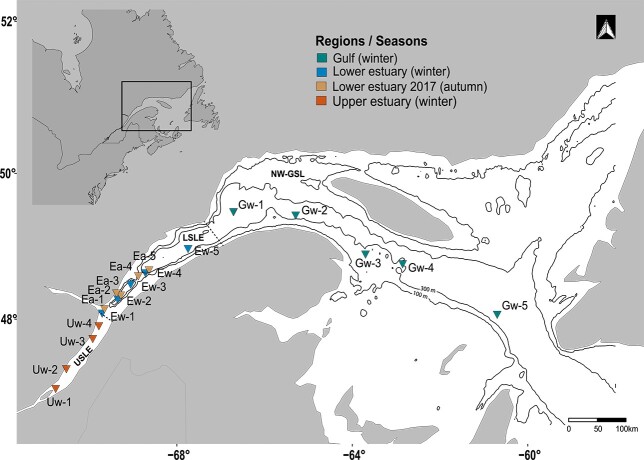
Fig. 1.
Map of the estuary and the Gulf of St. Lawrence, including sampling stations in autumn 2017 and winter 2018. The map was drawn with R v4.1.1, using open source data. Bathymetry was provided by the National Oceanic and Atmospheric Administration (NOAA) throughout the marmap package.
In the Mediterranean Sea, where most of the knowledge on the feeding ecology of B. arctica has been documented, B. arctica is a generalist and opportunistic feeder, due to its wide range of food sources (Cartes, 2011). This species has been described as a non-selective omnivorous-carnivorous feeder (e.g. Cartes and Sorbe, 1998; Cartes, 2011). Based on gut contents and fatty acid trophic markers B. arctica seems to feed on phytodetritus up to large zooplankton species, including dinoflagellates, tintinnids, cnidarians and mesozooplankton such as Calanus species. Despite this large range of prey size, crustaceans seemed to be the main prey, suggesting a more carnivorous than detritus based feeding behavior (Mauchline, 1980; Cartes and Sorbe, 1998; Cartes, 2011). Like many macrozooplankton species exposed to variable food supply, B. arctica accumulate energetic reserves in the form of lipids. These are stored as neutral lipids, mostly build as triacylglycerol and/or wax esters that can be quickly metabolized for short-term energy requirements (Lee et al., 2006). According to Cartes (2011) the lipid content of B. arctica is higher during winter than summer. The same dynamics were also observed for Thysanoessa species in the St. Lawrence. For krill, such dynamic are expected to be the result of an energetic strategy to partially limit the need for food when primary production is at its lowest, potentially limiting intra- and interspecific competition when resource availability is low (Cabrol et al., 2019a, 2019b). However unlike krill species little is known about the feeding habits and the physiological strategies of B. arctica and how this species might acclimate to local variation of food supply, especially in winter in the St. Lawrence system.
This study aims to quantify spatiotemporal variations of trophic ecology of B. arctica focusing on the total lipid content and composition as well as their trophic level along a transect of 1000 km from the upper part of the St. Lawrence estuary to the Cabot Strait in the Gulf of St. Lawrence with a special focus on the winter season. The specific objectives were to examine the spatial and seasonal variation of (i) energetic reserves (e.g. lipid dynamics) of B. arctica, and (ii) its trophic position to explore potential changes. We used a multimarker approach, combining lipid class analyses (Parrish and Ackman, 1985) and stable isotope analyses (Fry, 2006). In the present study, the physiological conditions of individuals of B. arctica were evaluated by measuring the total lipid content and lipid composition using thin layer chromatography, while trophic levels were calculated from nitrogen stable isotope ratios. These results were further compared with the three dominant krill species from the same area (Cabrol et al., 2019a, 2019b). In addition to serving as a baseline, these results will help to better understand the role of this deep-dwelling mysid species in the bentho-pelagic coupling in the subarctic St. Lawrence ecosystem.
METHODS
Sampling
B. arctica was collected in autumn 2017 and winter 2018 on board the research vessel R.V. Coriolis II and the Canadian coast guard icebreaker CCGS Amundsen, respectively. In autumn 2017 the study covered four sites in the LSLE as part of two distinct expeditions (Fig. 1). In winter 2018, sampling was extended into the GSL, covering a transect of 14 stations (>1000 km) from Québec to Cabot Strait (Fig. 1). B. arctica was collected with a 1 m diameter ring net with 202 μm mesh size equipped with a strobe light (Jacknet), however, no open-closing device was attached to the net. In autumn 2017, oblique tows were taken by lowering the net in an angle of 60° and a speed of 1 m.s−1 down to 10 m off bottom and retrieving it in the same manner. Due to malfunctioning of the flowmeter, only a qualitative analysis of the zooplankton has been performed. In winter 2018, vertical hauls were carried out from 5 m off bottom to the surface at a speed of 0.5 m.s−1. B. arctica were counted and sorted on board. For every station specimens were individually frozen at −80°C for lipid class and stable isotope analyses, respectively (see Table 1 for sample size). Water samples for particulate organic matter (POM) were collected at two discrete depths (10 m and 10 m from the bottom) using a rosette sampler system equipped with 12 L Niskin-type bottles. However, only the bottom sample was used in the present study. To determine stable isotopes of POM, two technical replicates of 1 to 2 L of water were filtered through pre-combusted and pre-weighed 21 mm GF/F filters. The filters were stored at −80°C before analyses.
Table 1.
Station coordinates of sampling stations in the Estuary and Gulf of St. Lawrence in autumn 2017 and winter 2018, number of Boreomysis arctica captured and/or analyzed for lipid classes and stable isotopes
| Mission | Year | Season | Station | Latitude | Longitude | Station depth (m) | Total caught (n) | Lipid class analyses—(n) | Stable isotope analyses (n) |
|---|---|---|---|---|---|---|---|---|---|
| Coriolis II | 2017 | Fall | Ea-1 | 48° 10.550 | 69° 29.750 | 226 | no count | 5 | 6 |
| Ea-2 | 48° 23.300 | 69° 15.100 | 309 | no count | 6 | 21 | |||
| Ea-3 | 48°22.399 | 69° 09.712 | 260 | no count | 4 | 10 | |||
| Ea-4 | 48°37.729 | 68° 45.184 | 338 | no count | 0 | 10 | |||
| Ea-5 | 48°42.937 | 68° 31.332 | 345 | no count | 6 | 0 | |||
| CCGS | 2018 | Winter | Uw-1 | 47°01.937 | 70°45.914 | 18 | 0 | 0 | 0 |
| Amundsen | Uw-2 | 47°18.776 | 70°31.005 | 22 | 0 | 0 | 0 | ||
| Uw-3 | 47°44.145 | 69°54.871 | 143 | 0 | 0 | 0 | |||
| Uw-4 | 47°55.012 | 69°46.741 | 127 | 0 | 0 | 0 | |||
| Ew-1 | 48°06.472 | 69°34.143 | 125 | 0 | 0 | 0 | |||
| Ew-2 | 48°18.305 | 69°12.839 | 196 | 15 | 6 | 10 | |||
| Ew-3 | 48°30.703 | 68°55.196 | 272 | 47 | 3 | 10 | |||
| Ew-4 | 48°40.0950 | 68°34.9286 | 330 | 66 | 5 | 10 | |||
| Ew-5 | 49°00.111 | 67°36.643 | 295 | 23 | 5 | 9 | |||
| Gw-1 | 49°31.983 | 66°10.723 | 340 | 16 | 6 | 10 | |||
| Gw-2 | 49°27.569 | 65°09.429 | 370 | 9 | 3 | 4 | |||
| Gw-3 | 48°55.349 | 63°34.081 | 330 | 16 | 5 | 10 | |||
| Gw-4 | 48°47.316 | 62°43.452 | 340 | 3 | 0 | 0 | |||
| Gw-5 | 48°05.515 | 60°34.129 | 420 | 1 | 0 | 0 | |||
| Total B. arctica analyzed | 54 | 110 | |||||||
Lipid class analyses
Lipid analyses were performed on the entire individual. Frozen individuals of similar size range (mean total length of 1.92 ± 0.35 cm) were directly extracted in dichloromethane:methanol by grinding using a modified Folch procedure (Chen et al., 1981) as described in detail in Parrish et al. (1999). Then, lipid content and class composition were determined using flame ionization detection system using silica gel-coated chromarods (S-V Chromarods; Shell-USA; see Parrish, 1987 for details). Every lipid extracts were scanned using an Iatroscan (Mark-VI, Iatron Laboratories) to separate aliphatic wax esters, ketones, triacylglycerols, alcohols, sterols, acetone mobile polar lipids, and phospholipids (Parrish et al., 1999). However, the method used cannot differentiate steryl esters from wax esters (Parrish, 2013) and are therefore grouped in the term SE-WE and we referred in the following to this group as wax esters for simplification. In fact, in marine animals, a many store lipids as wax esters (Budge et al., 2006) justifying this simplification. For each lipid class, Sigma standards were used for post quantification. Chromatograms were analyzed using the integration software Peak Simple version 3.2 (SRI). Total lipids are expressed as μg.mg−1 of wet weight and correspond to the sum of all classes (Parrish, 2013). Lipid classes were expressed as relative concentration in percent of the total lipids (% of total lipids).
Stable isotope analysis
To estimate trophic levels, stable isotopes of nitrogen (δ15N) of B. arctica and POM were determined by a continuous-flow Isotope Ratio Mass Spectrometry using a Deltaplus XP mass spectrometer (ThermoScientific) coupled with an elemental analyzer COSTECH 4010 (Costech Analytical). Certified reference material Sorghum Flour Standard OAS (B2159) and High Organic Sediment Standard OAS (B2151) and two in-house standards Caffeine and Mueller Hint Broth were used as internal standards for nitrogen calibration. Data analysis was performed with Isodat 3.0. Analytical error of measurement was 0.2‰ for δ15N of B. arctica and Calanus spp. and was 0.4‰ for POM-filters. Stable isotopes are expressed in δ notation as the deviation from international standards in parts per thousand according to the following equation:
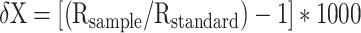 |
(1) |
where X is 15N, and R is the corresponding ratio 15N/14N.
Trophic level determination
The trophic level (TL) was calculated using the equation of Post (2002):
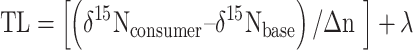 |
(2) |
Where δ15Nconsumer = δ15N of B. arctica, δ15Nbase = δ15N of POM near to the bottom and λ is the TL of the baseline used, i.e. TL = 1 for mean POM per season and region. Δn is the estimated trophic discrimination factor for nitrogen. We used deep-water POM signatures that were averaged for each region and season (please refer to Fig. 5 for isotopic values) because B. arctica is known to be a deep dwelling species. As no trophic discrimination factors were available for B. arctica a trophic discrimination factor of 2‰ was chosen for nitrogen according to McCutchan et al. (2003) and Chew et al. (2012).
Fig. 5.
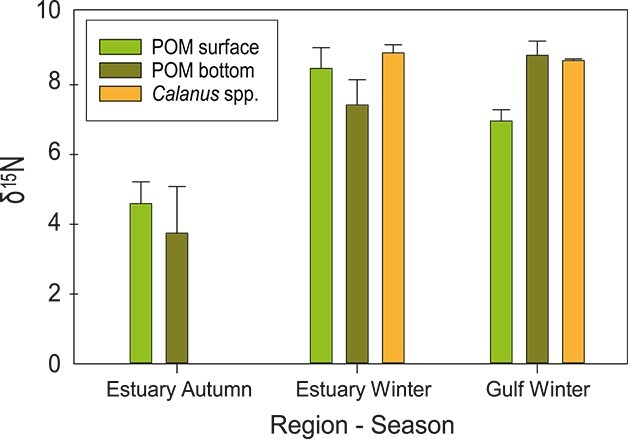
Mean nitrogen stable isotopes (δ15N) of particulate organic matter (POM) from the surface and the bottom layer, as well as of Calanus spp. in the estuary in autumn 2017 and winter 2018 and the Gulf of St. Lawrence in winter 2018.
Statistical analyses
To test differences in lipid contents and trophic levels within and between the two seasons and among sampling sites, univariate two-way PERMANOVAs were performed with “season” (2 levels) and “station” (11 levels) as factors, using an Euclidean distance, type III error structures and 9 999 permutations (Anderson et al., 2008). To determine if the lipid class composition varied between seasons and sampling regions, a multivariate two-way PERMANOVA was carried out, based on an Euclidian distance matrix with untransformed data (Clarke et al., 2014; Clarke and Gorley, 2015). In addition, pairwise multiple comparison tests were used to identify differences among factors when necessary. A non-metric dimensional scaling (n-MDS) was used on the Euclidian distance matrix to visualize inter-individual, inter-station and seasonal variation of lipid class composition. The similarity percentages (SIMPER) procedure was performed on untransformed data to identify specific lipid classes explaining the most important dissimilarities of significant pairwise comparisons. All the tests mentioned above were performed on PRIMER version 7.0.21 (Clarke and Gorley, 2015) with PERMANOVA (Anderson et al., 2008).
RESULTS
Abundance and distribution of B. arctica
In winter, B. arctica were absent in the upper St. Lawrence Estuary (USLE) and at the head of the Laurentian Channel (Ew-1), but were found downstream throughout the lower St. Lawrence Estuary (LSLE) and the Gulf of St. Lawrence (GSL; Fig. 2). Mean abundances were two times higher in the LSLE (40 ind. m−2) than in the GSL (Fig. 2), however not significantly different (t-test, P = 0.06). Highest abundances of 60 and 84 ind.m−2 were found at Ew-3 and Ew-4, respectively, in the LSLE, whereas most of the stations in the GSL showed abundance, <15 ind.m−2 (Fig. 2).
Fig. 2.
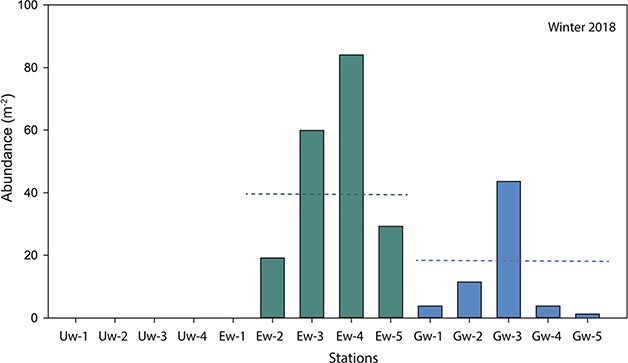
Abundance of Boreomysis arctica sampled during winter 2018 in the lower estuary (Ew) and the Gulf (Gw) of St. Lawrence. Data for 2017 were not included due to malfunctioning of the flowmeter. Dashed line represents the mean of each region.
Total lipid content and lipid class composition
Total lipid content of B. arctica was higher in winter than in autumn (Fig. 3A). Spatial variability of total lipid content was high, but in winter 2018 lipid content generally increased from the LSLE to the GSL showing the highest mean total lipid content at Gw-2 of 94.55 ± 12.78 mg.g−1 WW (Fig. 3A). Compared to the three dominant krill species in winter, highest mean lipid contents of B. arctica were most similar to that of T. inermis and higher than that of T. raschii and M. norvegica (Fig. 3A). However, when comparing the mean winter lipid content over all stations of 65.44 ± 20.00 mg.g−1 WW of B. arctica, it is comparable to that of T. raschii.
Fig. 3.
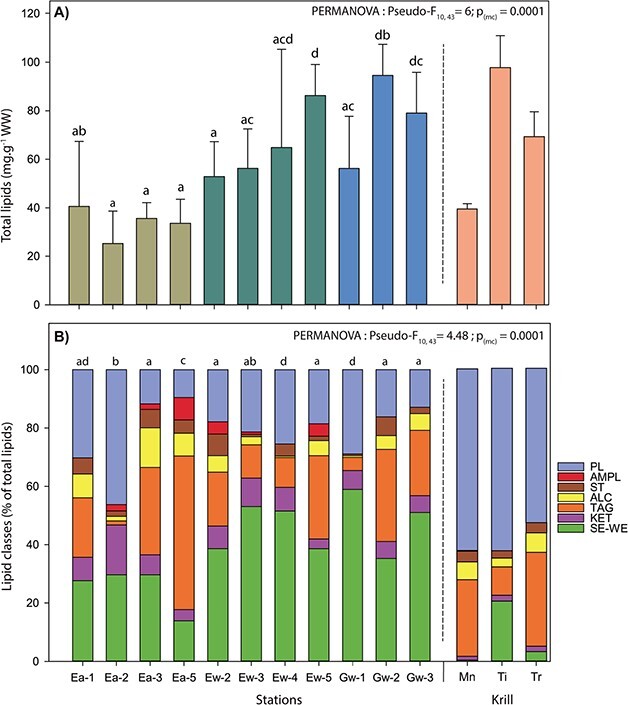
Lipid content in mg.g−1 of WW (A) and lipid classes in percent of total lipids (B) for Boreomysis arctica sampled in the estuary and the Gulf of St. Lawrence in autumn 2017 and winter 2018. Note that lipid content and composition of the dominant krill species have been added for comparison and were extracted from Cabrol et al., 2019a sampled during winter 2015. Mn = Meganyctiphanes norvegica; Ti = Thysanoessa inermis and Tr = Thysanoessa raschii.
Lipid composition of B. arctica was highly variable among individuals and among stations (Fig. S1). It differed among stations in autumn, while in winter it was more homogenous throughout the EGSL (Fig. 3B). In winter, lipid composition of most of the individuals tend to be comparable with a dominance of wax esters (SE-WE) and similar proportions of triacylglycerol (TAG) and polar lipids (PL) (Fig. 3B). The differences between autumn and winter were mainly related to different proportions of SE-WE, TAG and PL (71–88% cumulative contribution—SIMPER), showing high proportions of TAG and PL in autumn (26% and 25% respectively, LSLE) compared to high contribution of SE-WE in winter (44% LSLE and 51% GSL).
Trophic level of B. arctica
The trophic levels of B. arctica varied spatially and temporally (Fig. 4A and B). More depleted δ15N ratios were found in autumn compared to winter (Fig. 4A), whereas the trophic levels of B. arctica showed a contrasting pattern, being higher (3.2 to 4.3) in autumn than in winter (2.0 to 2.7; Fig. 4B). Compared to krill species in the EGSL in winter, B. arctica was positioned lower than all three krill species in winter (Fig. 4B). Lower trophic levels (Fig. 5), POM showed enriched δ15N ratios of > 2‰ in winter compared to autumn (ANOVA, Fdf2 = 12.455, P < 0.001), that were comparable with zooplankton, the calanoid copepod Calanus spp. (ANOVA, Fdf3 = 0.714, P = 0.556).
Fig. 4.
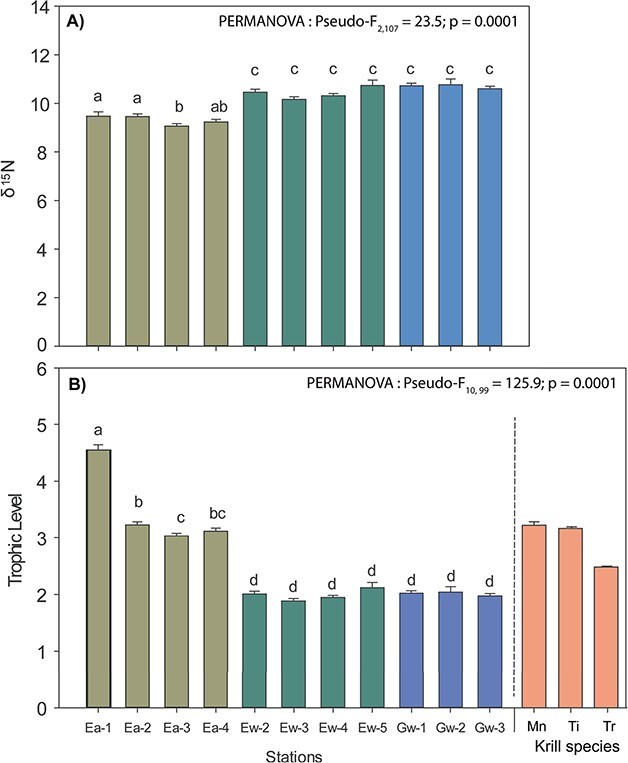
Nitrogen stable isotopes (A) and trophic level (B) of Boreomysis arctica in autumn 2017 and winter 2018 in the estuary and the Gulf of St. Lawrence. Note that trophic levels of krill sampled in the LSLE during winter 2015 were added for comparison (Cabrol et al., 2019a). LSLE = Lower St. Lawrence estuary; Mn = Meganyctiphanes norvegica; Ti = Thysanoessa inermis and Tr = Thysanoessa raschii.
DISCUSSION
Abundance and distribution of B. arctica
As expected, B. arctica were absent from shallow and brackish part of the St. Lawrence Estuary. As B. arctica has never been observed at depth shallower than 150 m in the LSLE (Harvey et al., 2009), results suggest that B. arctica does not pass the shallow sill (40 m) at the head of the Laurentian Channel to migrate into the USLE. In winter, B. arctica were found consistently throughout the LSLE and the GSL, showing a tendancy of higher abundance in the LSLE than in the GSL. These abundances suggest that B. arctica is an important contributor to the macrozooplankton community besides the three dominant krill species, Meganycthiphanes novegica, T. raschii and T. inermis also occurring in high abundance in the St. Lawrence ecosystem (Harvey et al., 2009, McQuinn et al. 2015). Unfortunately, due to the flowmeter breakdown abundance data in autumn 2017 were not available, preventing any comparison between the two seasons. In 2018, the winter abundances were lower than mean abundances of spring and fall combined with values of 124 ± 10 ind.m−2 and 128 ± 7 ind.m−2 found in the early 2000s in the LSLE and the north western GSL, respectively (Descroix et al., 2005). Theses authors showed that abundances of B. arctica did not vary significantly among years and seasons either in the LSLE or the GSL (Descroix et al., 2005). Data availability is too limited to determine if lower abundances are related to season or potentially to changes in time over the 20 years between the two sampling periods. Over these 20 years, the physico-chemical conditions of the deep-water habitat have, however, changed. Water temperature has increased around 1 to 2°C (Galbraith, 2006; Galbraith et al., 2020) and oxygen condition has severely deteriorated with the increase of the hypoxia level over the last 80 years (Gilbert et al., 2005; Jutras et al., 2020, 2023). This environmental modification is expected to have consequences for the biology and the life cycle of the deep dwelling mysid B. arctica, but without proper knowledge on physiological tolerance to temperature and oxygen of this species, this potential impact cannot be estimated.
Body condition
We estimated physiological body condition in terms of total lipid content. Lipid content of B. arctica showed temporal and spatial variability, being higher in winter than in autumn. Bamstedt (1977) found in the Norwegian Korsfjorden that temporal lipid dynamics in B. arctica revealed highest lipid percentages of dry weight from October to February and lowest values in early June. Differences of lipid dynamics of B. arctica between these two ecosystems may be due to particular climatologies of environmental parameters that may have an influence on the phenology of B. arctica. It has been suggested that B. arctica in the Mediterranean produce at least two generations per year (Cartes and Sorbe, 1998), showing a peak of recruits in late winter-early spring and one in autumn. Thus, the low lipid content in autumn individuals of the EGSL, might indicate a loss in energy due to reproduction, whereas high lipid content in winter (February) might be indicative of a pre-reproduction period before late winter–spring spawning occurs. However, this explanation should be taken with care, as neither sex nor the developmental stage of the B. arctica used in the present study is known.
Compared to the three dominant krill species, lipid dynamics in B. arctica seemed to be similar to Meganycthyphanes norvegica, showing low lipid content in autumn and highest lipid content in winter (Cabrol et al., 2019a, b). However, the total lipid content in B. arctica in winter was higher than that of M. norvegica, and similar to levels observed for Thysanoessa species. Lipid dynamics are distinct from the three krill species, B. arctica accumulating a higher amount of energy from autumn to winter than M. norvegica. This stands in contrast to the energetic reserve utilization over winter when compared to both Thysanoessa species (Cabrol et al., 2019a). This dynamic also suggests that B. arctica is able to acquire enough food from autumn to winter to store energetic lipid reserves. Results of lipids class composition are supporting this hypothesis, as the proportion of energy reserve lipids, composed mainly by wax esters (SE-WE) and triacylglycerol (TAG) in B. arctica, were higher in winter than in autumn. The variability in lipid composition of B. arctica among stations was higher in autumn when compared to the winter throughout the EGSL. In winter, lipid composition of all the individuals tends to be comparable with a dominance of SE-WE and similar proportions of TAG and polar lipids. The spatial variability in autumn might be related to spatial heterogeneity in food supply and/or food selectivity, resulting in different nutritional histories increasing the variability of the physiological condition among individuals. For example, B. arctica at Ea-2 had the lowest lipid content characterized by large TAG depletion and a high proportion of polar lipids, regulating membrane composition (Sargent et al., 1993; Tocher et al., 2008). This depletion of energy reserves indicates weak physiological condition that could be related to energy loss through reproduction, stressful conditions or dying individuals. In contrast, B. arctica collected at Ea-5 showed similar low total lipid content, but largely higher proportion of TAG, which might have been acquired by feeding on a high proportion of phytoplankton and/or zooplankton as seen in krill species (Cabrol et al. 2019a, b). Cartes (2011) also suggested the presence of a high inter-individual variability in food selection even among individuals collected at the same sampling site, indicating that differential feeding strategies might occur among co-occurring specimens. Accordingly, our results stress the importance of the large intraspecific variability in both energy content and lipid composition between B. arctica individuals, particularly at some stations. In these sites, inter-individual variations within B. arctica were similar and even higher to the ones found among the three dominant krill species of the northern Atlantic (Cabrol et al., 2019a). High intra-specific variability is a common feature of opportunistic species (Bolnick et al., 2003; Cabrol et al., 2019a, b, 2021). However, such high levels suggest that individuals seem to acclimate to face adverse condition in food supply, as the variability observed probably results from distinct trophic and/or life histories among individuals. As a consequence, physiological condition estimated by lipid content and/or composition and related to their fitness could be expected to vary from one individual to another. In winter, feeding conditions might be more restricted and homogeneous in terms of availability and diversity, reducing the inter-individual physiological condition of B. arctica. Early stages and small copepod species abundance might be strongly reduced in winter, whereas diapausing stages of large copepods such as Calanus spp. might be easily available in the deep-water layer. Diapausing Calanus spp. are non-migrating stages that aggregate in the warmer deep-water layer (150–300 m) compared to the upper freezing surface layer from autumn to spring (Plourde et al., 2002, 2003). This increases the encounter probability of Calanus spp. with B. arctica during winter. Diapausing Calanus spp. have accumulated high amounts of reserve lipids during summer and autumn, mainly in the form of WE (Scott et al., 2002), to fulfill the reduced metabolic demand of the dormancy over winter (Maps et al., 2010, 2011, 2012). Thus, diapausing Calanus spp. could be the most important prey, supplying B. arctica with WE and might explain the net increase of lipid content as well as the proportion of WE (~1.5 to 2 times) observed during the winter at all stations.
Trophic level of B. arctica
Although B. arctica is present throughout the Laurentian Channel of the EGSL, the position in the pelagic food web and the trophic relationships of B. arctica are not known. This species is expected to be dependent on resource transport into the deep water of the Laurentian Channel through the sedimentation of POM (phytoplankton, detritus) and diel vertical migrating or diapausing zooplankton. Thus, feeding conditions will be strongly dependent on spatial–temporal variation in primary and secondary production. In the present study, we determined the trophic levels of B. arctica in autumn 2017 and winter 2018 throughout the Laurentian Channel by δ15N ratios and confirmed spatial and temporal variations with depleted δ15N ratios in autumn compared to winter. However, the trophic levels of B. arctica showed a contrasting pattern, as the trophic position showed higher levels in autumn, compared with winter, including high spatial variability in the EGSL. These results suggest that B. arctica was mostly carnivorous in autumn, whereas it exhibits a more omnivorous feeding behavior in winter. B. arctica in the western Mediterranean and the Algerian Basin showed relatively low δ15N ratios of 6.5 ± 0.8 and 6.3 ± 0.8, respectively, when compared to the suprabenthic community suggesting an omnivorous filter feeding (Madurell et al., 2008; Fanelli et al., 2009). Evidence of an omnivorous to carnivorous feeding habit was observed based on FAs markers (Cartes, 2011) and gut content analyses (Cartes and Sorbe, 1998) in the Mediterranean Sea. Considering the limited amount of phytoplankton available in winter, carnivory would be the strategy of choice. However, we found the opposite. This might be partly due to the calculation of the trophic level based on δ15N of POM. POM showed enriched δ15N ratios of > 2‰ in winter compared to autumn, indicative of highly degraded POM in winter. The degradation process metabolizes preferentially lighter isotopes enriching the POM in δ15N (Fry, 2006). In consequence, when bottom POM δ15N ratios from each region and season were used to calculate the trophic levels of B. arctica, these results might artificially decrease their trophic levels, particularly in winter. Alternatively, benthic feeding might also occur, which is known for several mysid species (Mauchline, 1980). Unfortunately, no recent data on the benthos community is available, as the last assessment published is from 2006 (Steinhart, 2023), and since then the hypoxic conditions extended spatially into the GSL and deteriorated severely (Jutras et al., 2023), so that it is unknown to what extend benthic food would be available to B. arctica.
Compared to krill species in the EGSL, B. arctica was positioned higher than all three krill species in autumn. M. norvegica and T. inermis were considered as true carnivorous feeder, based on fatty acid and stable isotope ratios (Cabrol et al., 2019a, 2019b), so that carnivorous feeding is also suggested for B. arctica in autumn. In contrast, the trophic level of B. arctica in winter was comparable to that of T. raschii, however, lipid composition was very different, indicating that both species in spite of similar low trophic levels, have a different feeding behavior and physiology.
CONCLUSION
For the first time, we determined physiological condition, lipid composition and trophic levels of the deep dwelling mysid B. arctica in autumn and in winter under sea ice conditions in the Estuary and Gulf of St. Lawrence. B. arctica showed high total lipid contents suggesting that B. arctica was able to acquire sufficient food to store energy reserves mostly in the form of wax ester particularly in winter. Potential prey organisms such as lipid-rich copepods, in particular, Calanus, stay in the deep-water layers of the EGSL during the winter diapause, increasing the encounter probability with B. arctica. In view of the biological carbon pump, B. arctica potentially acts as an important organism sequestering carbon produced in the upper water layers to the deep layer. In the food web, B. arctica is very rich in energetic lipids compared to the three dominant krill species in the EGSL. Therefore, B. arctica is probably an interesting prey from an energetic point of view for pelagic and bentho-pelagic consumers that have the enzymatic machinery necessary to digest or assimilate wax esters. However, to better understand the trophic relationships among B. arctica and their prey, further quantitative studies (e.g. fatty acid trophic markers and isotopic mixing models) are needed to quantify and better qualify the diet of B. arctica and its role in the EGSL ecosystem. Finally, although the aerobic capacities of B. arctica are unknown, it appears very interesting that B. arctica is still present in similar abundances than 15 years ago, despite the increase of hypoxia in the deep water layer of the Laurentian Channel. Hypoxia might present a restraining environmental variable to this species. However, there is some indication from a congener species Boreomysis oparva in the Pacific Ocean that tolerates well hypoxic conditions, even providing a refuge from predators (Saltzman, 1996). In the wake of the environmental changes intensifying in the EGSL, it would be interesting to increase the understanding of the ecology and the fate of this abundant and energy-rich species.
Supplementary Material
Acknowledgement
We thank the RQM for the opportunity to participate in the Winter Expedition on the Canadian icebreaker CCGS Amundsen. Special thanks are extended to G. Cervello, D. Ouellet and C. Marcil for their invaluable assistance in the laboratory and the field. We extend special thanks to M. Babin for his help stable isotope analysis and to C. Hammer for revising the English language and manuscript improvement.
Contributor Information
Gesche Winkler, Institut de Sciences de la Mer, Université du Québec à Rimouski, Québec-Océan, 310 Allée des Ursulines, G5L3A1, Rimouski, Quebec, Canada.
Jory Cabrol, Maurice Lamontagne Institute, Fisheries and Oceans Canada, 850 Rte de la Mer, G5H 3Z4, Mont-Joli, QC, Canada.
Réjean Tremblay, Institut de Sciences de la Mer, Université du Québec à Rimouski, Québec-Océan, 310 Allée des Ursulines, G5L3A1, Rimouski, Quebec, Canada.
FUNDING
This research was supported by the discovery grant from the Natural Sciences and Engineering Research Council of Canada to G.W. and R.T. and by the program “Odyssée Saint-Laurent” of the “Réseau Québec maritime” (RQM). This is a contribution to the research cluster “Quebec–Ocean”.
Data Availability
All used datasets for this study including stable isotopes and lipid composition can be found in the SLGO-St. Lawrence Global Observatory or can be provided upon request to the corresponding author.
References
- Anderson, M. J., Gorley, R. N. and Clarke, K. R. (eds.) (2008) PERMANOVA for PRIMER: guide to software and statistical methods. PRIMER-E., 8, 39–53. 10.1016/j.hal.2008.08.017. [DOI] [Google Scholar]
- Bamstedt, U. (1977) Studies on the deep-water pelagic community of Korsfjorden, western Norway. Sarsia, 63, 145–154. 10.1080/00364827.1978.10411332. [DOI] [Google Scholar]
- Benkort, D., Plourde, S., Winkler, G., Cabrol, J., Ollier, A., Cope, L. E. and Maps, F. (2019) Individual-based modeling explains the contrasted seasonality in size, growth, and reproduction of the sympatric Arctic (Thysanoessa raschii) and Nordic krill (Meganyctiphanes norvegica) in the St. Lawrence estuary, eastern Canada. Limnol. Oceanogr., 64, 217–237. 10.1002/lno.11032. [DOI] [Google Scholar]
- Blais, M., Galbraith, P. S., Plourde, S., Devine, L. and Lehoux, C. (2021) Chemical and biological oceanographic conditions in the estuary and gulf of St. Lawrence during 2019 DFO. Can. Sci. Advis. Sec. Res. Doc., 2021/002 iv, 1–66. [Google Scholar]
- Bolnick, D. I., Svanback, R., Fordyce, J. A., Yang, L. H., Davis, J. M., Hulsey, C. D. and Forister, M. L. (2003) The ecology of individuals: incidence and implications of individual specialization. American Naturalist, 161, 1–28. [DOI] [PubMed] [Google Scholar]
- Brunel, P., Bossé, L. and Lamarche, G. (1998) Catalogue of the marine invertebrates of the estuary and gulf of Saint Lawrence. Can. Spec. Publ. Fish. Aquat. Sci., 126, 1–405. [Google Scholar]
- Budge, S. M., Iverson, S. J. and Koopman, H. N. (2006) Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Marine Mammal Sci., 22, 759–801. 10.1111/j.1748-7692.2006.00079.x. [DOI] [Google Scholar]
- Cabrol, J., Lesage, V., Leclerc, A., Giard, J., Bérubé, M., Michaud, R. and Nozais, C. (2021) Individual and population dietary specialization decline in fin whales during a period of ecosystem shift. Sci. Rep., 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrol, J., Nadalini, J.-B., Galbraith, P. S., Tremblay, R., Nozais, C., Starr, M., Plourde, S. and Winkler, G. (2019b) Seasonal and largescale spatial variability of the energy reserves and the feeding selectivity of Meganyctiphanes norvegica and Thysanoessa inermis in a subarctic environment. Prog. Oceanogr., 179, 102203. 10.1016/j.pocean.2019.102203. [DOI] [Google Scholar]
- Cabrol, J., Trombetta, T., Amaudrut, S., Aulanier, F., Sage, R., Tremblay, R., Nozais, C., Starr, M.. et al. (2019a) Trophic niche partitioning of dominant North-Atlantic krill species, Meganyctiphanes norvegica, Thysanoessa inermis and T. Raschii. Limnol. Oceanogr., 64, 165–181. 10.1002/lno.11027. [DOI] [Google Scholar]
- Cartes, J. E. (2011) Temporal changes in lipid biomarkers, especially fatty acids, of the deep-sea crustaceans Boreomysis arctica and Nematoscelis megalops: implications of their biological cycle and habitat near the seabed. J. Mar. Bio. Ass. U. K., 91, 783–792. 10.1017/S0025315410002018. [DOI] [Google Scholar]
- Cartes, J. E. and Sorbe, J. C. (1998) Aspects of population structure and feeding ecology of the deep-water mysids Boreomysis arctica, a dominant species in western Mediterranean slope assemblages. J. Plankton Res., 20, 2273–2290. 10.1093/plankt/20.12.2273. [DOI] [Google Scholar]
- Chen, I. S., Shen, C. S. J. and Sheppard, A. J. (1981) Comparison of methylene chloride and chloroform for the extraction of fats from food products. J. Am. Oil Chem. Soc., 58, 599–601. 10.1007/BF02672373. [DOI] [Google Scholar]
- Chew, L. W., Chong, V. C., Tanaka, K. and Sasekumar, A. (2012) Phytoplankton fuel the energy flow from zooplankton to small nekton in turbid mangrove waters. Mar. Ecol. Prog. Ser., 469, 7–24. 10.3354/meps09997. [DOI] [Google Scholar]
- Clarke, K. R. and Gorley, R. N. (eds.) (2015) PRIMER v7: User Manual/Tutorial, Plymouth, PRIMER-E. [Google Scholar]
- Clarke, K. R., Gorley, R. N., Somerfield, P. J. and Warwick, R. M. (eds.) (2014) Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd edn, Plymouth, PRIMER-E. [Google Scholar]
- Cope, L. E., Plourde, S. and Winkler, G. (2021) Seasonal variation of growth and reproduction of the subarctic krill species, Thysanoessa raschii, driven by environmental conditions in the estuary and gulf of St. Lawrence. J. Plankton Res., 43, 458–474. 10.1093/plankt/fbab032. [DOI] [Google Scholar]
- Descroix, A., Harvey, M., Roy, S. and Galbraith, P. S. (2005) Macrozooplankton community patterns driven by water circulation in the St. Lawrence marine system, Canada. Mar. Ecol. Prog. Ser., 302, 103–119. 10.3354/meps302103. [DOI] [Google Scholar]
- Fanelli, E., Cartes, J. E., Rumolo, P. and Sprovieri, M. (2009) Food-web structure and trophodynamics of mesopelagic - suprabenthic bathyal macrofauna of the Algerian Basin based on stable isotopes of carbon and nitrogen. Deep-Sea Research I, 56, 1504–1520. [Google Scholar]
- Fry, B. (2006). Stable Isotope Ecology (Vol. 521). New York: Springer, 10.1007/0-387-33745-8. [DOI] [Google Scholar]
- Fulton, R. S. (1982) Predatory feeding of two marine mysids. Mar. Biol., 72, 183–191. 10.1007/BF00396919. [DOI] [Google Scholar]
- Galbraith, P. S. (2006) Winter water masses in the Gulf of St. Lawrence. J. Geophys. Res., 111, C06022, 3159. 10.1029/2005JC00. [DOI] [Google Scholar]
- Galbraith, P. S., Chassé, J., Shaw, J.-L., Dumas, J., Caverhill, C., Lefaivre, D. and Lafleur, C. (2020) Physical oceanographic conditions in the Gulf of St. Lawrence during 2019. DFO Can. Sci. Advis. Sec. Res. Doc., 2020/030 iv +, 84. [Google Scholar]
- Gilbert, D., Sundby, B., Gobeil, C., Mucci, A. and Tremblay, G.-H. (2005) A seventy-two-year record of diminishing deep-water oxygen in the St. Lawrence estuary: the Northwest Atlantic connection. Limnol. Oceanogr., 50, 1654–1666. 10.4319/lo.2005.50.5.1654. [DOI] [Google Scholar]
- Harvey, M., Galbraith, P. S. and Descroix, A. (2009) Vertical distribution and diel migration of macrozooplankton in the St. Lawrence marine system (Canada) in relation with the cold intermediate layer thermal properties. Prog. Oceanogr., 80, 1–21. 10.1016/j.pocean.2008.09.001. [DOI] [Google Scholar]
- Jutras, M., Dufour, C. O., Mucci, A., Cyr, F. and Gilbert, D. (2020) Temporal changes in the causes of the observed oxygen decline in the St. Lawrence estuary. J. Geophys. Res., 125, e2020JC016577. 10.1029/2020JC016577. [DOI] [Google Scholar]
- Jutras, M., Mucci, A., Chaillou, G., Nesbitt, W. A. and Wallace, D. W. R. (2023) Temporal and spatial evolution of bottom-water hypoxia in the St Lawrence estuarine system. Biogeosciences, 20, 839–849. 10.5194/bg-20-839-2023. [DOI] [Google Scholar]
- Lee, R. F., Hagen, W. and Kattner, G. (2006) Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser., 307, 273–306. 10.3354/meps307273. [DOI] [Google Scholar]
- Macquart-Moulin, C. (1993) Répartition verticale, migrations et stratifications superficielles des mysidacés et amphipodes pélagiques Sur les marges méditerranéenne et Atlantique françaises. J. Plankton Res., 15, 1149–1170. 10.1093/plankt/15.10.1149. [DOI] [Google Scholar]
- Madurell, T., Fanelli, E. and Cartes, J. E. (2008) Isotopic composition of carbon and nitrogen of suprabenthic fauna in the NW Balearic Islands (western Mediterranean). Journal of Marine Systems, 71, 336–345. 10.1016/j.jmarsys.2007.03.006. [DOI] [Google Scholar]
- Maps, F., Pershing, A. J. and Record, N. R. (2012) A generalized approach for simulating growth and development in diverse marine copepod species. ICES J. Mar. Sci., 69, 370–379. 10.1093/icesjms/fsr182. [DOI] [Google Scholar]
- Maps, F., Plourde, S., Lavoie, D., McQuinn, I. and Chasse, J. (2014) Modelling the influence of daytime distribution on the transport of two sympatric krill species (Thysanoessa raschii and Meganyctiphanes norvegica) in the Gulf of St. Lawrence, eastern Canada. ICES J. Mar. Sci., 71, 282–292. 10.1093/icesjms/fst021. [DOI] [Google Scholar]
- Maps, F., Plourde, S., McQuinn, I. H., St-Onge-Drouin, S., Lavoie, D., Chassé, J. and Lesage, V. (2015) Linking acoustics and finite-time Lyapunov exponents reveals areas and mechanisms of krill aggregation within the Gulf of St. Lawrence, eastern Canada. Limnol. Oceanogr., 60, 1965–1975. 10.1002/lno.10145. [DOI] [Google Scholar]
- Maps, F., Plourde, S. and Zakardjian, B. (2010) Control of dormancy by lipid metabolism in Calanus finmarchicus: a population model test. Mar. Ecol. Prog. Ser., 403, 165–180. 10.3354/meps08525. [DOI] [Google Scholar]
- Maps, F., Runge, J. A., Leising, A., Pershing, A. J., Record, N. R., Plourde, S. and Pierson, J. J. (2011) Modelling the timing and duration of dormancy in populations of Calanus finmarchicus from the Northwest Atlantic shelf. J. Plankton Res., 34, 36–54. [Google Scholar]
- Mauchline, J. (1980) The biology of mysids and euphausids (Crustacea, Mysidacea). Adv. Mar. Biol., 18, 3–317. [Google Scholar]
- McCutchan, J. H., Lewis, W. M., Kendall, C. and McGrath, C. C. (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos, 102, 378–390. 10.1034/j.1600-0706.2003.12098.x. [DOI] [Google Scholar]
- McQuinn, I. H., Plourde, S., St. Pierre, J.-F., Dion, M. (2015) Spatial and temporal variations in the abundance, distribution, and aggregation of krill (Thysanoessa raschii and Meganyctiphanes norvegica) in the lower estuary and Gulf of St. Lawrence. Progr. Oceanogr., 131, 159–176. 10.1016/j.pocean.2014.12.014. [DOI] [Google Scholar]
- Ollier, A., Chabot, D., Audet, C. and Winkler, G. (2018) Metabolic rates and spontaneous swimming activity of two krill species (Euphausiacea) under different temperature regimes in the St. Lawrence estuary, Canada. J. Crustac. Biol., 38, 697–706. 10.1093/jcbiol/ruy028. [DOI] [Google Scholar]
- Parrish, C. C. (1987) Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by Iatroscan flame ionization detection. Can. J. Fish. Aquat. Sci., 44, 722–731. 10.1139/f87-087. [DOI] [Google Scholar]
- Parrish, C.C., Peltékian E., Dickson G., Epstein A.L., Garcia L. (1999). Determination of total lipid, lipid classes, and fatty acids in aquatic samples, In Arts, M.T. and Wainman, B.C. [eds.], Lipids in Freshwater Ecosystems . Springer; New York. pp. 173–180. 10.1023/A:1008022713466, 30 [DOI] [Google Scholar]
- Parrish, C. C. (2013) Lipids in marine ecosystems, Lipids in marine ecosystems. ISRN Oceanogr, 2013, 1–16. 10.5402/2013/604045. [DOI] [Google Scholar]
- Parrish, C. C. and Ackman, R. G. (1985) Calibration of the Iatroscan-Chromarod system for marine lipid class analyses. Lipids, 20, 521–530. 10.1007/BF02534893. [DOI] [Google Scholar]
- Plourde, S., Dodson, J. J., Runge, J. A. and Therriault, J.-C. (2002) Spatial and temporal variations in copepod community structure in the lower St. Lawrence estuary. Canada. Mar. Ecol. Prog. Ser., 230, 211–224. 10.3354/meps230211. [DOI] [Google Scholar]
- Plourde, S., Joly, P., Runge, J. A., Dodson, J. and Zakardjian, B. (2003) Life cycle of Calanus hyperboreus in the lower St Lawrence estuary and its relationship to local environmental conditions. Mar. Ecol. Prog. Ser., 255, 219–233. 10.3354/meps255219. [DOI] [Google Scholar]
- Plourde, S., Lehoux, C., McQuinn, I. H. and Lesage, V. (2016) Describing krill distribution in the western North Atlantic using statistical habitat models. DFO Can. Sci. Advis. Sec. Res. Doc., 2016/111 v +, 34. [Google Scholar]
- Plourde, S., Winkler, G., Joly, P., St-Pierre, J. F. and Starr, M. (2011) Long-term seasonal and interannual variations of krill spawning in the lower St. Lawrence estuary, Canada, 1979−2009. J. Plankton Res., 33, 703–714. 10.1093/plankt/fbq144. [DOI] [Google Scholar]
- Post, D. M. (2002) Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology, 83, 703–718. 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2. [DOI] [Google Scholar]
- Saltzman, J. (1996) Ecology and life history traits of the benthopelagic mysid Boreomysis oparva from the eastern tropical Pacific oxygen minimum zone. Mar. Ecol. Prog. Ser., 139, 95–103. 10.3354/meps139095. [DOI] [Google Scholar]
- Sargent, J. R., Bell, J. G., Bell, M. V., Henderson, R. J. and Tocher, D. R. (1993) The metabolism of phospholipids and polyunsaturated fatty acids in fish. In Callou, B. and Vittelo, P. (eds.), Coastal and Estuarine Studies – Aquaculture: Fundamental and Applied Research, Am, Geo, Union, Washington, pp. 103–124. [Google Scholar]
- Scott, C. L., Kwasniewski, S., Falk-Petersen, S. and Sargent, J. R. (2002) Species differences, origins and functions of fatty alcohols and fatty acids in the wax esters and phospholipids of Calanus hyperboreus, Calanus glacialis and Calanus finmarchicus from Arctic waters. Mar. Ecol. Prog. Ser., 235, 127–134. 10.3354/meps235127. [DOI] [Google Scholar]
- Simard, Y., Lacroix, G. and Legendre, L. (1986) Diel vertical migrations and nocturnal feeding of a dense coastal krill scattering layer (Thysanoessa raschi and Meganyctiphanes norvegica) in stratified surface waters. Mar. Biol., 91, 93–105. 10.1007/BF00397575. [DOI] [Google Scholar]
- Simard, Y. and Lavoie, D. (1999) The rich krill aggregation of the Saguenay – St. Lawrence Marine Park: hydroacoustic and geostatistical biomass estimates, structure, variability, and significance for whales. Can. J. Fish. Aquat. Sci., 56, 1182–1197. 10.1139/f99-063. [DOI] [Google Scholar]
- Steinhart, R. (2023) Temporal and Spatial Analysis of the Relationship between Hypoxia, Temperature, and Benthic Biodiversity in the St. Lawrence Estuary and Gulf MSc thesis, Memorial University, St. John’s, Canada. [Google Scholar]
- Tattersall, W. M. and Tattersall, O. S. (1951) The British Mysidacea, Royal Society, London. [Google Scholar]
- Tocher, D. R., Bendiksen, E. A., Campbell, P. J. and Bell, J. G. (2008) The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture, 280, 21–34. 10.1016/j.aquaculture.2008.04.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All used datasets for this study including stable isotopes and lipid composition can be found in the SLGO-St. Lawrence Global Observatory or can be provided upon request to the corresponding author.