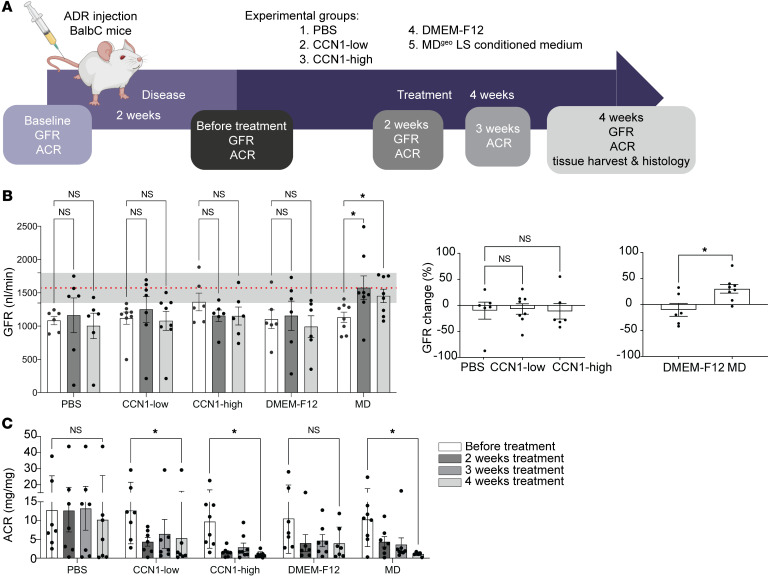
Figure 8. Treatment with MD biologicals improves kidney function in CKD.
(A) Illustration of therapeutic study design for testing the effects of MD biologicals (human recombinant CCN1 and LS-conditioned MDgeo cell culture media) using the adriamycin (ADR) model of glomerulosclerosis in BALB/c mice. (B) Time course of the absolute (left) and relative (normalized to baseline before treatment, right) changes in GFR followed in the same mice measured by the MediBeacon noninvasive transcutaneous method. Note the significant improvement of GFR returning to normal baseline levels (the red dotted line represents mean ± SEM [gray shaded area], measured at baseline) in the MD treatment group indicating functional regression of FSGS pathology; n = 6–8. (C) Time course of albuminuria (albumin/creatinine ratio [ACR]) changes followed in the same mice measured by ELISA. Note the significant improvement in albuminuria in the CCN1 and MD treatment groups in contrast to the PBS and DMEM-F12 controls; n = 6–8. Data represent mean ± SEM. *P < 0.05, ****P < 0.0001, 2-way (mixed-effect) ANOVA with Tukey’s test (B, left, and C), 1-way ANOVA followed by Dunnett’s test (B, center), or t test (B, right).