Abstract
Acute decompensated heart failure (ADHF) treatment leads to significant hemodynamic changes. The aim of our study was to quantitatively analyze the dynamics of mitral regurgitation (MR) severity (evaluated by transthoracic echocardiography) which occur during the treatment of ADHF and to correlate these changes with the clinical condition of patients as well as heart failure biochemical markers. The study included 27 consecutive adult patients (40.7% females, mean age 71.19 ± 11.2 years) who required hospitalization due to signs of acute HF. Echocardiographic assessment was performed upon admission and discharge together with clinical and laboratory evaluation. Significant reduction in dyspnea intensity [0–100 scale] (81.48 ± 9.07 vs. 45.00 ± 11.04 pts, p < 0.001), body weight (84.98 ± 18.52 vs. 79.77 ± 17.49 kg, p < 0.001), and NT-proBNP level (7520.56 ± 5288.62 vs. 4949.88 ± 3687.86 pg/ml, p = 0.001) was found. The severity of MR parameters decreased significantly (MR volume 44.92 ± 22.83 vs. 30.88 ± 18.77 ml, p < 0.001; EROA 0.37 ± 0.17 vs. 0.25 ± 0.16 cm2, p < 0.001; VC 6.21 ± 1.48 vs. 5.26 ± 1.61 mm, p < 0.001). Left atrial area (35.86 ± 9.11 vs. 32.47 ± 9.37, p < 0.001) and mitral annular diameter (42.33 ± 6.63 vs. 39.72 ± 5.05. p < 0.001) also underwent statistically significant reductions. An increase in LVEF was observed (34.73 ± 13.88 vs. 40.24 ± 13.19%, p < 0.001). In 40.7% of patients, a change in MR severity class (transition from a higher class to a lower one) was observed: 6/8 (75%) patients transitioned from severe to moderate and 6/18 (33.3%) patients transitioned from moderate to mild class. Treatment of ADHF leads to a significant reduction in MR severity, together with significant reductions in left atrial and mitral annular dimensions. Quantitative measurement of MR dynamics offer valuable assistance for ADHF management.
Keywords: Heart failure, Mitral valve, Mitral regurgitation, Echocardiography
Introduction
Acute decompensated heart failure (ADHF) is defined as a sudden worsening of the signs and symptoms of heart failure, and is often a potentially life-threatening condition requiring hospitalization and emergency therapy [1]. Long-term, optimal treatment of heart failure significantly improves patient prognosis by reversing the unfavorable remodeling of the left ventricular myocardium [2].
Mitral valve regurgitation is frequently observed in patients hospitalized for ADHF and is associated with significant dilatation of the left ventricular cavity and mitral annular distension. Severe mitral valve insufficiency diagnosed on admission due to ADHF symptoms is associated with an unfavorable prognosis [3]. Pharmacotherapy used in ADHF treatment has a significant impact on the dynamics of mitral valve insufficiency [4–6]. It has been shown that optimal treatment of acute heart failure, using diuretics and vasodilators, leads to reductions in left ventricular filling pressure and systemic vascular resistance, and thus, significantly reduces mitral regurgitation [4]. Moreover, it has been demonstrated that this phenomenon occurs mainly in the mechanism of mitral ring diameter reduction and decreased left ventricular end-diastolic dimension, both of which lead to a reduction in the regurgitant orifice area [7].
Because the severity of mitral regurgitation may significantly change during pharmacological treatment of ADHF, mitral valve evaluation by echocardiographic imaging could have a significant impact on the qualification of patients for surgical management of mitral valve disease. Nevertheless, there are only a few studies describing the changes in cardiac hemodynamics during ADHF management and the impact of these changes on long-term prognosis [8]. Therefore, the aim of our study was to quantitatively analyze changes in mitral regurgitation severity, as evaluated by transthoracic echocardiography, which occur during the treatment of ADHF and to correlate these changes with the clinical condition of patients as well as with heart failure biochemical markers.
Materials and methods
Study population
The study included 27 consecutive adult patients (40.7% females, mean age 71.19 ± 11.2 years) requiring hospitalization due to signs and symptoms of acute heart failure and were admitted to the Department of Cardiac and Vascular Diseases and Department of Diagnostics, Jagiellonian University Medical College, John Paul II Hospital in Kraków, Poland. Exclusion criteria were: fever, infection or sepsis upon admission, confirmed diagnosis of concomitant acute coronary syndrome, acute heart failure caused by significant cardiac arrhythmias (tachycardia or bradycardia), hyperthyroidism/hypothyroidism, pregnancy and puerperium.
Patient management and evaluation
All enrolled patients received conventional pharmacological treatment in accordance with current guidelines for the treatment of acute heart failure [9]. Inclusion into the study did not result in disruption of therapy, which was determined solely by the attending physician.
The standard diagnostic scheme was used in all patients, which included an assessment of basic vital sign parameters (heart and respiration rate, peripheral oxygen saturation and arterial blood pressure). Resting electrocardiogram was performed upon admission. Body mass and fluid balance was monitored daily. Basic biochemical tests were performed. Additionally, serum NT-proBNP level was determined on the first day of treatment and upon discharge. A subjective assessment of clinical symptoms (severity of dyspnea) was also performed using a scale from 0 to 100 points.
Echocardiographic assessment
Per-protocol transthoracic echocardiography was performed in all patients on admission and on the day of discharge. All examinations were performed by one researcher with several years of experience in performing echocardiographic examinations using the Philips iE33 and Philips Epiq ultrasound machines (Philips Healthcare, Eindhoven, Netherlands). Additionally all examinations were supervised by the head of Echocardiography Department. Post-processing and study evaluation were performed using a dedicated workstation. All linear measurements were performed using virtual calipers. Echocardiographic assessment of heart structure and mitral regurgitation was performed according to current guidelines [10].
The following parameters were analyzed: left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) dimensions; left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volume; left ventricular ejection fraction (LVEF, calculated using the Simpson biplane method); left atrial dimension (measured in the parasternal long axis view); left atrial surface (measured in the apical four-chamber view); mitral annular diameters (measured in the parasternal long axis and apical four-chamber views); mitral inflow parameters (peak E-wave and A-wave velocity, E/A ratio). In cases of atrial fibrillation, measurements from 3 to 5 heart rates were obtained and mean values were calculated. The assessment of mitral regurgitation severity was carried out based on the following parameters: semi-quantitative assessment using Vena Contracta (VC) measurement; quantitative assessment using the flow convergence (FC) method; Proximal Isovelocity Surface Area (PISA) radius; effective regurgitant orifice area (EROA); and mitral regurgitant volume (MR Vol).
Statistical analysis
Data are presented as mean ± standard deviation (SD) with the median and interquartile ranges (Q1, Q3) for continuous variables or percentages (%) for categorical variables. Distribution analysis of the studied data was performed using the Kolmogorov–Smirnov test. The Student’s t-test and Mann–Whitney U test were used for statistical comparisons. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using StatSoft STATISTICA 13.1 software (StatSoft Inc., Tulsa, OK, USA).
This study was approved by the Bioethical Committee of the Jagiellonian University in Kraków, Poland (No 122.6120.294.2015). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all patients.
Results
All enrolled patients initially presented with symptoms of significant dyspnea (either NYHA class III or IV). Ischemic etiology of heart failure was found in 48% of patients. The average subjective severity of dyspnea (assessed on a 0–100 point scale) was 81.5 ± 9.07 pts. Detailed clinical characteristics of patients on admission are presented in Table 1. Most of the included patients had previously received standard treatment for chronic heart failure. Angiotensin-converting enzyme inhibitors or aldosterone receptor blockers were used in 70.4% of patients, while all patients were chronically treated with beta-blockers (Table 1). Seven patients from the study population required treatment with vasopressors and inotropes, while none required the use of an intra-aortic balloon pump or mechanical ventilation. The level of NT-proBNP was high in all patients with a mean value of 7520.0 ± 5645.25 pg/ml. Atrial fibrillation was noted in 74.1% of patients, including persistent or permanent atrial fibrillation in 5.4% of patients. No significant differences in terms of patient sex were found in any of the baseline clinical parameters. The average daily dosages of administrated diuretics, ACE-I, MRA and beta-blockers are presented in Table 2. During ADHF treatment, patient body mass and NT-proBNP level decreased significantly, together with a significant decrease in dyspnea (Table 3).
Table 1.
Clinical characteristics of the study group on admission (n = 27)
| Clinical parameter | Value at baseline |
|---|---|
| Age (years) | 71.2 ± 11.2 (range 41.0–90.0) |
| BMI | 30.2 ± 5.4 (range 22.2–41.2) |
| QRS duration (ms) | 116.2 ± 27.0 (range 80.0–160.0) |
| Arterial hypertension (%) | 92.6 |
| Diabetes mellitus (%) | 37.0 |
| Atrial fibrillation (%) | 74.1 |
| COPD (%) | 7.4 |
| Coronary artery disease (%) | 48.1 |
| Previous myocardial infarction (%) | 37.0 |
| Previous CABG/PCI (%) | 40.7 |
| LBBB in ECG (%) | 15.4 |
| RBBB in ECG (%) | 11.5 |
| Chronic treatment with ACE-I (%) | 70.4 |
| Chronic treatment with beta-blockers (%) | 100 |
| Chronic treatment with MRA (%) | 70.4 |
Data are expressed as the mean value ± standard deviation and range for continuous variables and as a percentage of patients for qualitative variables
ACE-I angiotensin-converting enzyme inhibitors, BMI ody mass index, CABG oronary artery bypass grafting, COPD chronic obstructive pulmonary disease, MRA aldosterone receptor antagonists, PCI percutaneous coronary intervention
Table 2.
Mean daily doses of administrated diuretics, beta-blockers, ACEI and MRA
| At admission | At discharge | ||
|---|---|---|---|
| Beta-blockers (mg/24 h) | Metoprolol succinate | 43.75 ± 11.57 | 40.62 ± 12.93 |
| Bisoprolol | 3.36 ± 2.41 | 4.46 ± 2.97 | |
| Carvedilol | 14.58 ± 9.54 | 12.5 ± 10.82 | |
| Nebivolol | 3.75 ± 1.76 | 1.87 ± 0.88 | |
| ACEI (mg/24 h) | Perindopril | 3.75 ± 1.76 | 3.75 ± 1.76 |
| Ramipril | 4.68 ± 2.86 | 3.21 ± 1.44 | |
| MRA (mg/24 h) | Spironolactone | 31.66 ± 11.44 | 37.5 ± 13.17 |
| Eplerenone | 37.5 ± 14.43 | 34.72 ± 15.02 | |
| Diuretics (mg/24 h) | Furosemide | 101.48 ± 79.40 | 90.40 ± 46.23 |
| Torasemide | 8.70 ± 13.97 | 25.19 ± 41.19 |
Data are expressed as the mean value ± standard deviation
ACEI angiotensin-converting enzyme inhibitors, MRA aldosterone receptor antagonists
Table 3.
Change in heart failure parameters during the treatment period
| Parameter | Value at baseline | Value at discharge | Value change (baseline-discharge) | p-value |
|---|---|---|---|---|
| Level of dyspnea (0–100 pts) | 81.48 ± 9.07 (range 60–90) | 45 ± 11.04 | 36.54 | < 0.001 |
| Body weight (kg) | 84.98 ± 18.52 | 79.77 ± 17.49 | 5.21 | < 0.001 |
| NT-proBNP (pg/ml) | 7520.56 ± 5288.62 | 4949.88 ± 3687.86 | 2570.68 | 0.001 |
Data are expressed as the mean value ± standard deviation
Echocardiographic parameters are presented in Table 4. Initially, patients showed significant remodeling of the left ventricular myocardium (mean LVEDV = 179.1 ± 80.51 ml) and significant dilatation of the mitral valve annulus (mean 42.3 ± 6.63 mm). Moreover, left ventricular systolic function was significantly reduced (mean LVEF = 34.73 ± 13.88%). Mitral regurgitation was assessed as severe in 29.6% of patients. In our patients mitral regurgitation was connected to left ventricular remodeling and mitral annulus dilation.
Table 4.
Change in echocardiographic parameters during the treatment period (n = 27)
| Parameter | Value at baseline | Value at discharge | Value change (baseline-discharge) | P-value |
|---|---|---|---|---|
| LA diameter PLAX (mm) | 54.81 ± 6.57 | 50.77 ± 6.32 | 4.04 | < 0.001 |
| LA area in AP4 (cm2) | 35.86 ± 9.11 | 32.47 ± 9.37 | 3.38 | < 0.001 |
| LVEDD in PLAX (mm) | 61± 11.41 | 58.35 ± 11.03 | 2.65 | < 0.001 |
| LVESD in PLAX (mm) | 48.35 ± 13.47 | 46.65 ± 13.27 | 1.69 | 0.002 |
| LVEDV (Simpson biplane method) (ml) | 179.08 ± 80.51 | 174.15 ± 80.03 | 4.92 | 0.307 |
| LVEF (Simpson biplane method) (%) | 34.73 ± 13.88 | 40.24 | 5.50 | < 0.001 |
| Mitral annular diameter in PLAX (mm) | 42.33 ± 6.63 | 39.72 ± 5.05 | 2.61 | < 0.001 |
| Mitral annular diameter in AP4 (mm) | 42.46 ± 4.99 | 39.67 ± 5.13 | 2.79 | < 0.001 |
| Mitral mean gradient (mmHg) | 2.25 ±1.31 | 1.81 ± 0.89 | 0.45 | 0.02 |
| Peak E-wave velocity (cm/s) | 122.83 ± 31.29 | 107.33 ± 23.61 | 15.50 | < 0.001 |
| E/A ratio | 0.95 ± 1.22 | 0.94 ± 1.16 | 0.004 | 0.98 |
| VC (mm) | 6.21 ± 1.48 | 5.26 ± 1.61 | 0.95 | < 0.001 |
| PISA MR radius (mm) | 7.84 ± 2.65 | 6.36 ± 2.25 | 1.04 | < 0.001 |
| MR VTI (cm) | 136.32 ± 24.67 | 136.37 ± 23.84 | -0.05 | 0.98 |
| MR V max (m/s) | 4.61 ± 0.63 | 4.53 ± 0.65 | 0.07 | 0.4 |
| MR EROA (cm2) | 0.37 ± 0.17 | 0.25 ± 0.16 | 0.11 | < 0.001 |
| MR volume (ml) | 44.92 ± 22.83 | 30.88 ± 18.77 | 14.04 | < 0.001 |
Data are expressed as the mean value ± standard deviation
AP4 apical four chambers view EROA-effective regurgitant orifice area, LA left atrium, LVEDD left ventricular end diastolic diameter, LVEDV left ventricular end diastolic volume, LVEF left ventricular ejection fraction, LVESD left ventricular end systolic diameter, LVESV left ventricular end systolic volume, MR mitral regurgitation, PISA proximal Isovelocity Surface Area, PLAX parasternal long axis view, VC vena contracta, VTI velocity time integral
A significant decrease in the severity of mitral regurgitation echocardiographic parameters was observed, especially in MR volume (31% reduction), EROA (32% reduction), and VC (15% reduction) (Table 4). Left atrial and mitral annular dimensions also underwent a statistically significant reduction (Table 4). Left ventricular ejection fraction increased from 34 to 40%. Nevertheless, no significant changes in LVEDV, velocity time integral (MR VTI), or maximum velocity of the mitral return wave (MR Vmax) were noted (Table 4).
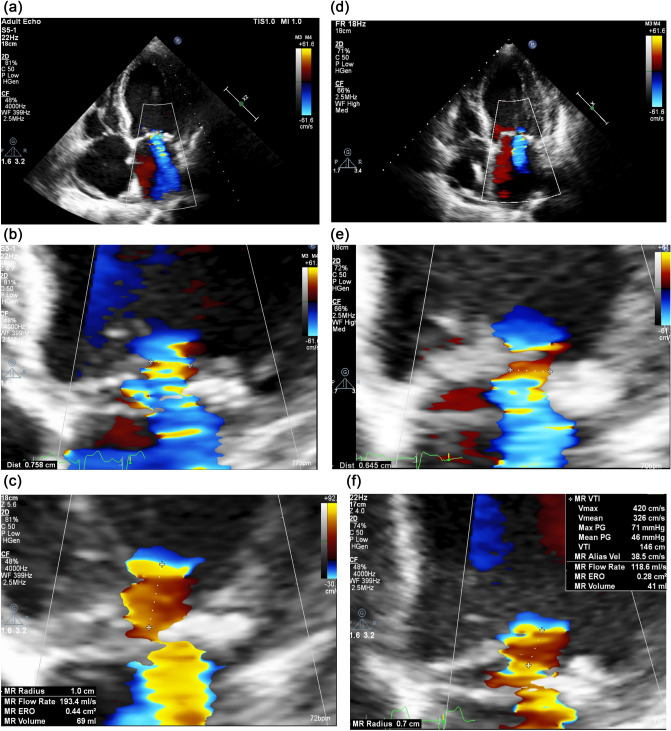
In 40.7% of patients, a change in mitral regurgitation severity (transition from one class to another) was observed during the hospitalization period (Table 5). Specifically, 75.0% of patients with severe mitral regurgitation at baseline had improved to moderate, while 33.3% of patients with an initially moderate wave had improved to mild (Table 5). An exemplary echocardiographic assessment of changes in the severity of mitral regurgitation is presented in the Fig. 1.
Table 5.
Categorical changes in the severity of mitral regurgitation and a comparison of baseline and discharge number of patients in each group (mild, moderate, severe)
| Severity of mitral regurgitation | Baseline n (%) | Discharge n (%) |
|---|---|---|
| Mild | 1 (3.7) | 7 (25.9) |
| Moderate | 18 (66.7) | 18 (66.7) |
| Severe | 8 (29.6) | 2 (7.4) |
| Baseline | Discharge | |
| Mild 1 patient | Mild 1 patient | |
| Moderate 0 patients | ||
| Severe 0 patients | ||
| Mild 6 patients | ||
| Moderate 18 patients | Moderate 12 patients | |
| Severe 0 patients | ||
| Mild 0 patients | ||
| Moderate 6 patients | ||
| Severe 8 patient | Severe 2 patients | |
n number of patients in each category
Fig. 1.
Images showing changes in the severity of mitral regurgitation during the treatment of ADHF. Initially severe mitral regurgitation—a, b and c in echocardiographic assessment on discharge was evaluated as moderate—(d), (e) and (f)
Discussion
The main finding of the present study is to show significant quantitative reduction of mitral regurgitation in patients treated due to ADHF. In 40% of patients, the reduction led to a change in mitral regurgitation severity class, which may be the most important factor in the decision-making process concerning surgical treatment of such patients.
The results of our current study demonstrate the dynamic nature of mitral regurgitation in patients with ADHF. Until now, studies describing mitral regurgitation severity during the treatment of acute cardiovascular decompensation, as assessed by echocardiography, were mainly based on semi-quantitative measurements. In our work, apart from semi-quantitative measurements, results from quantitative assessment of the mitral regurgitation are also presented. The reduction in mitral regurgitation severity during ADHF treatment is associated with a combination of many different factors such as a reduction in afterload, pressure gradient change between the left ventricle and left atrium, and a reduction in left ventricular and mitral annular dimensions, which are associated with a reduction in EROA [7]. In line with previous observations involving groups of patients treated for acute heart failure [7], our study also showed a significant reduction in the dimensions of the left atrium, mitral annular diameter, and EROA, assessed using the PISA method, when comparing baseline measurements with discharge measurements. On the other hand, no significant reduction was recorded in the LVEDV when assessed by the biplanar method. This could be related to the baseline characteristics of the study population, which was affected by advanced left ventricular remodeling at baseline.
The presence of significant ischemic mitral regurgitation in patients with a history of myocardial infarction and heart failure with reduced ejection fraction significantly worsens the long-term prognosis, regardless of the LVEF, age, and NYHA functional class [11, 12]. In contrast to primary mitral regurgitation, there is still no clear evidence whether a reduction in secondary mitral regurgitation is associated with improved survival [13]. Nevertheless, minimally invasive procedures such as the percutaneous edge-to-edge repair system (MitraClip) are commonly used, which can provide a reduction in the severity of heart failure symptoms and are associated with a reduction in unfavorable remodeling of the left ventricular myocardium [14]. As shown in the COAPT trial, the appropriate selection of heart failure patients for the MitraClip procedure leads to a reduction in the number of hospitalizations and decreased mortality in the 24-month follow-up [15].
Our study illustrates the importance of a solid understanding of mitral regurgitation dynamics in heart failure patients in everyday clinical practice. Importantly, our study showed a significant reduction in mitral regurgitation severity (8 patients with severe regurgitation at baseline vs. 2 patients at discharge) as a result of standard treatment for cardiovascular decompensation. This may have a significant impact on the selection of management strategies in patients, including the decision to perform surgical treatment for valvular disease. In chronic severe secondary mitral regurgitation (SMR) valve surgery is often associated with high risk of complications. Indications for isolated valve surgery are of course restrictive. We consider surgery in patient with concomitant coronary artery disease who are candidates for CABG. In other cases of SMR persisting severe despite optimal treatment (including CRT if indicated) we can consider transcatheter edge-to-edge repair. Quantification of functional mitral regurgitation should always be performed after stabilization of heart failure decompensation and reduction of fluid overload.
It is also worth mentioning that in the group of patients with initially severe mitral regurgitation admitted to the hospital due to symptoms of ADHF, mitral regurgitation reduction to moderate or mild classes do not necessarily imply an improved prognosis. This was demonstrated in the group of patients with ADHF and dynamic mitral regurgitation initially meeting the criteria of a severe wave, which then significantly reduces during treatment process, where the prognosis is much worse than in the patients with insignificant regurgitation, but at a similar level as in the group of patients with persistent severe mitral regurgitation [3]. Therefore, patients with dynamic and severe mitral regurgitation also require greater attention and further follow-up to introduce a suitable treatment, including surgical management if needed.
Limitations of the study
A limitation of our study is the fact that it is a single-center analysis with a relatively small study group, involving patients diagnosed with ischemic (48%) and non-ischemic (52%) heart dysfunction. However, we believe that due to the high statistical significance of the observed changes, this does not interfere with our quantitative, qualitative, and clinical analyses of mitral regurgitation associated with ADHF.
Conclusions
Standard pharmacological treatment in ADHF patients leads to a significant reduction in mitral valve severity expressed as a reduction in mitral regurgitation volume, EROA, and vena contracta width. As a result of treatment, most of the patients with severe mitral regurgitation were then classified as having moderate mitral regurgitation. A significant reduction in left atrial and mitral annular dimensions were also observed. Quantitative measurement of mitral regurgitation dynamics could offer valuable information for the management of ADHF patients. Observed changes of mitral insufficiency may also serve for future studies evaluating length of the treatment of ADHF and tailoring the therapy to the individual patient.
Author contributions
WP and KB designed the study and were in charge of overall direction and planning. KB performed all echocardiographic examinations. MK, PR, MKH and PB contributed to data acquisition, statistical analysis and preparation of the text of the publication. The authors read and approved the final manuscript.
Funding
The study was supported by Jagiellonian University Medical College (Grant No. K/2DS/007820).
Availability of data and material
Data are available to researchers on request for purposes of reproducing the results by directly contacting the corresponding author.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Bioethical Committee of the Jagiellonian University in Kraków, Poland (No. 122.6120.294.2015). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kurmani S, Squire I. Acute heart failure: definition, classification and epidemiology. Curr Heart Fail Rep. 2017;14(5):385–392. doi: 10.1007/s11897-017-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fanarow GC, Child JS, Laks H, Walden JA. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol. 1993;72(9):672–676. doi: 10.1016/0002-9149(93)90883-E. [DOI] [PubMed] [Google Scholar]
- 3.Kubo S, Kawase Y, Hata R, Maruo T, Tada T, Kadota K. Dynamic severe mitral regurgitation on hospital arrival as prognostic predictor in patients hospitalized for acute decompensated heart failure. Int J Cardiol. 2018;273:177–182. doi: 10.1016/j.ijcard.2018.09.093. [DOI] [PubMed] [Google Scholar]
- 4.Varriale P, David W, E. Chryssos B, Hemodynamic response to intravenous enalaprilat in patients with severe congestive heart failure and mitral regurgitation. Clin Cardiol. 1993;16(3):235–238. doi: 10.1002/clc.4960160314. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton MA, Stevenson LW, Child JS, Moriguchi JD, Woo M. Acute reduction of atrial overload during vasodilator and diuretic therapy in advanced congestive heart failure. Am J Cardiol. 1990;65(18):1209–1212. doi: 10.1016/0002-9149(90)90975-7. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam U, Bitar F, Akhter MW, Khan S, Patrus S, Derakhshani M. Intravenous nitroglycerin in the treatment of decompensated heart failure: Potential benefits and limitations. J Cardiovasc Pharmacol Ther. 2004;9(4):227–241. doi: 10.1177/107424840400900403. [DOI] [PubMed] [Google Scholar]
- 7.Rosario LB, Stevenson LW, Solomon SD, Lee RT, Reimold SC. The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: Importance of reduction in the regurgitant orifice size. J Am Coll Cardiol. 1998;32(7):1819–1824. doi: 10.1016/S0735-1097(98)00461-6. [DOI] [PubMed] [Google Scholar]
- 8.Ramasubbu K, Deswal A, Chan W, Aguilar D, Bozkurt B. Echocardiographic changes during treatment of acute decompensated heart failure: insights from the ESCAPE trial. J Card Fail. 2012;18(10):792–798. doi: 10.1016/j.cardfail.2012.08.358. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200m. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Victor MA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103(13):1759–1764. doi: 10.1161/01.CIR.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 12.Kajimoto K, Minami Y, Otsubo S, Sato N. Ischemic or nonischemic functional mitral regurgitation and outcomes in patients with acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol. 2017;120(5):809–816. doi: 10.1016/j.amjcard.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H, Falk V, Bax JJ, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 14.D’Ascenzo F, Moretti C, Marra WG, et al. Meta-analysis of the usefulness of mitraclip in patients with functional mitral regurgitation. Am J Cardiol. 2015;116(2):325–331. doi: 10.1016/j.amjcard.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307–2318. doi: 10.1056/nejmoa1806640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to researchers on request for purposes of reproducing the results by directly contacting the corresponding author.