Abstract
Fusarium wilt, caused by (Fusarium udum Butler), is a significant threat to pigeonpea crops worldwide, leading to substantial yield losses. Traditional approaches like fungicides and resistant cultivars are not practical due to the persistent and evolving nature of the pathogen. Therefore, native biocontrol agents are considered to be more sustainable solution, as they adapt well to local soil and climatic conditions. In this study, five isolates of F. udum infecting pigeonpea were isolated from various cultivars and characterized morphologically and molecularly. The isolate from the ICP 8858 cultivar displayed the highest virulence of 90%. Besides, 100 endophytic bacteria, 100 rhizosphere bacteria and three Trichoderma spp. were isolated and tested against F. udum isolated from ICP 8858 under in vitro conditions. Out of the 200 bacteria tested, nine showed highest inhibition, including Rb-4 (Bacillus sp.), Rb-11 (B. subtilis), Rb-14 (B. megaterium), Rb-18 (B. subtilis), Rb-19 (B. velezensis), Eb-8 (Bacillus sp.), Eb-11 (B. subtilis), Eb-13 (P. aeruginosa), and Eb-21 (P. aeruginosa). Similarly, Trichoderma spp. were identified as T. harzianum, T. asperellum and Trichoderma sp. Notably, Rb-18 (B. subtilis) and Eb-21 (P. aeruginosa) exhibited promising characteristics such as the production of hydrogen cyanide (HCN), cellulase, siderophores, ammonia and nutrient solubilization. Furthermore, treating pigeonpea seedlings with these beneficial microorganisms led to increased levels of key enzymes (POD, PPO, and PAL) associated with resistance to Fusarium wilt, compared to untreated controls. In field trials conducted for four seasons, the application of these potential biocontrol agents as seed treatments on the susceptible ICP2376 cultivar led to the lowest disease incidence. Specifically, treatments T2 (33.33) (P. aeruginosa) and T3 (35.41) (T. harzianium) exhibited the lowest disease incidence, followed by T6 (36.5) (Carbendizim), T1 (36.66) (B. subtilis), T4 (52.91) (T. asperellum) and T5 (53.33) (Trichoderma sp.). Results of this study revealed that, P. aeruginosa (Eb-21), B. subtilis (Rb-18) and T. harzianum can be used for plant growth promotion and management of Fusarium wilt of pigeonpea.
Keywords: Defence enzymes, Field trials, PGPR, P. aeruginosa, Trichoderma
Subject terms: Microbiology, Plant sciences
Introduction
Pigeonpea (Cajanus cajan (L.) Millsp.) holds a crucial position as a significant legume pulse crop globally, particularly in Southern and Eastern Africa, Asia, and South America, where it plays a major role in supporting the livelihoods of subsistence farmers1. In India pigeonpea cultivated in 45 Lha, with annual production of 42 Lt and contributing nearly 90% of world’ acreage and production2. Despite its importance, the crop faces considerable challenges, especially from biotic stresses, with Fusarium wilt caused by Fusarium udum being a major threat and causing substantial yield losses3,4. Fusarium wilt exhibits patchy symptoms during both seedling and adult stages, with yield losses varying depending on the stage of infection, ranging from 100% at the prepodding stage to 67% at pre-harvest and 30% at maturity. In severe cases, grain yield losses can reach up to 100% 5–7. The pathogenic F. udum resides in the soil, entering plants through root tips and disrupting water and mineral transport in vascular bundles. Initial symptoms include interveinal chlorosis and reduced leaf turgidity, progressing to distinctive features like a purple band spreading upward from the stem base and longitudinally split open stems displaying brown discoloration of vascular tissues5,8,9. Current management strategies primarily rely on chemical fungicides, but their effectiveness is limited and impractical for established crops due to pathogens soil borne nature. Concerns about fungicide resistant pathogens underscore the urgent need for sustainable and ecofriendly alternatives. A promising approach involves utilizing beneficial microbes as a substitute or complement to chemical management10,11. Beneficial microbes have the potential to combat pathogens and promote plant growth, offering valuable contributions to disease control and increased crop yields. Additionally, the success of biological control agents is often higher when they originate from the local environment, such as rhizosphere microbes and endophytes, compared to foreign microorganisms. Native microorganisms are well adapted to specific local conditions, including climate, soil characteristics, and soil microbiota. Notable examples of beneficial rhizosphere and endophytic microbes include Bacillus spp., Pseudomonas spp., and Trichoderma spp. In the rhizosphere, Trichoderma spp. act as effective biocontrol agents against soil borne pathogens, reducing F. udum populations and mitigating pigeonpea wilt through mechanisms like mycoparasitism, lytic enzyme production, nutrient competition, and the secretion of pathogen fighting secondary metabolites12–14. These interactions also impact plant biochemistry, leading to increased lignin deposition, higher phenol levels, and changes in enzyme activity in response to pathogen attacks15.
In both the rhizosphere and as endophytic bacteria, Bacillus spp. and Pseudomonas spp. employ various strategies to combat plant diseases, including antibiosis, lytic enzymes, resource competition, extracellular proteins, antifungal antibiotics, lipopeptides, siderophores, and hydrogen cyanide (HCN) production16. Additionally, these bacteria enhance nutrient availability to plants by mobilizing essential minerals such as phosphorus, potassium, and zinc through the production of organic acids17–19. Furthermore, Bacillus spp. and Pseudomonas spp. utilize induced systemic resistance (ISR) as a crucial mechanism to protect plants from specific diseases20,21. ISR involves altering cell wall structure and producing phytoalexin rich glycoproteins, pathogenesis related (PR) proteins, and hydroxyproline rich glycoproteins22 Plant growth promoting rhizobacteria (PGPR) strains contribute by generating antioxidant enzymes such as peroxidase (POD), phenylalanine ammonia lyase (PAL), and polyphenol oxidase (PPO), which serve as triggers for ISR in plants 22. Peroxidase is essential for processes like lignification, suberization, and the synthesis of phenols and glycoproteins, strengthening the plant cell wall and preventing fungal invasion23–25. Phenylalanine ammonia lyase, the initial enzyme in the phenylpropanoid pathway, is involved in the production of phytoalexins, phenols, and lignin. Bacillus spp. and Pseudomonas spp. enhance chitinase, PAL, PPO, Superoxide dismutase, and β-1,3-glucanase activity while inhibiting the production of polymethyl galacturonase by F. udum in pigeonpea26.
In the context of our study, we highlight the importance of utilizing native biocontrol agents, both fungal and bacterial, isolated from the rhizosphere and within plant tissues. These native bioagents offer distinct advantages, as they are well adapted to local soil and climatic conditions. Fertile alluvial soils with high organic matter in Bihar soils favour the growth of bioagents that can effectively manage wilt diseases.
Materials and methods
Seed material
Pigeonpea seeds of different cultivars were obtained under AICRP on (All India Coordinated Research Project) Pigeonpea wilt programme from IIPR (Indian Institute of Pulse Research) Khanpur.
Collection, isolation and characterization of the pathogen
Pigeonpea plants exhibiting typical wilt symptoms were collected from highly susceptible cultivars (ICP2376 and BAHAR), moderately resistant cultivar (ICP 8862) and resistant cultivars (ICP8858 and ICP9174) at the AICRP on Pigeonpea wilt disease sick plot located at Tirhut College of Agriculture, Dholi (25° 59′ 41.9″ N latitude and 85° 35′ 43.3″ E longitude). Stem segments showing vascular discoloration were collected, surface sterilized [(70% alcohol (30 s), 1% sodium hypochlorite (30 s) and sterile distilled water (3 × 60 s)] inoculated to Potato Dextrose Agar (PDA) medium and then incubated at 25 ± 2 °C for 72 h27. Colonies exhibiting growth with characteristic Fusarium morphology were selected, subcultured, and grown on PDA medium following the methods outlined by28,29. Cultural characteristics, such as growth rate, growth pattern, mycelial color, pigmentation, radial growth, and zonation, were recorded after an 8 day incubation period. Microconidia and macroconidia morphology were observed after 8 and 15 days of incubation, respectively.
Pathogenicity test
To study the pathogenicity and identity of the isolated fungus as Fusarium, Koch's postulates were conducted on the susceptible Pigeonpea cultivar ICP2376. Purified Fusarium cultures were grown in 250 mL conical flasks containing 100 g of sorghum grains, which were autoclaved at 121.8 °C under 15 lb pressure for 15 min. Following inoculation, the cultures were incubated for 15 days. The prepared inoculum was then mixed with sterilized sandy loamy soil at a 1:4 ratio (pathogen to soil, w/w) and placed in 15 cm diameter plastic pots. Pigeonpea seeds were subjected to surface sterilization with a sodium hypochlorite solution for 2 min, followed by three rinses with sterile distilled water. Each plastic pot accommodated 10 seedlings, with a group of pots without the pathogen serving as a control30. Wilt symptoms were observed and documented 45 days after sowing.
Percent Disease Incidence (PDI) was calculated by the formula
Similarly, the Translation Elongation Factor 1-α gene (TEF1α) and Internal Transcribed Spacer region gene (ITS) of the Fusarium isolates were amplified, and the sequences were submitted to NCBI GenBank for further analysis and documentation.
Collection and isolation of biocontrol agents
Ten rhizosphere soil samples and plant samples were collected from the Samastipur and Muzaffarpur districts in Bihar, characterized by temperatures ranging from 20 to 40 °C and an annual average temperature of approximately 26 °C (Supplementary Fig. 1).
To isolate rhizobacteria and Trichoderma spp., 10 g of rhizosphere soil was mixed with 90 mL of sterile distilled water and serially diluted up to 10–631. From 10–4 to 10–6 dilutions, an aliquot of 0.1 mL soil microbial suspensions were evenly spread over Nutrient Agar, King’s B, and Trichoderma-specific medium (TSM) from Himedia Laboratories, India. Incubation was carried out at 28 ± 2 °C for bacteria and 25 ± 2 °C for Trichoderma spp. Distinct bacterial colonies, exhibiting diverse morphological characteristics, were chosen, purified, and preserved in a 20% glycerol solution for future use. Fungal colonies were examined for morphological differences under a compound microscope at 400 × magnification (Olympus, Cx-21i, Japan). Subsequently, individual colonies identified as Trichoderma spp. were subcultured and stored based on their morphological features.
For isolating endophytic bacteria, healthy pigeonpea plants were harvested at the flowering stage. One gram stem samples underwent surface sterilization [70% alcohol (30 s), 1% sodium hypochlorite (30 s), sterile distilled water (3 × 60 s)], and were ground using a mortar and pestle in 9 mL of sterile water32. The grounded samples were serially diluted to 10–8, and 0.1 mL aliquots from this dilution were plated on Nutrient agar and King’s B agar plates. Incubation was done at 28 ± 2 °C in a BOD incubator for 2–3 days. (Supplementary Fig. 2).
In vitro evaluation of fungal and bacterial biocontrol agents against F. udum
The dual culture technique was employed to evaluate the antagonistic effects of bacterial and fungal isolates against F. udum isolated from Pigeonpea cultivar ICP 8858. For fungal evaluation, 5 mm mycelial discs of seven days old F. udum were positioned on one side of a petriplate, while 5 mm discs of seven day old Trichoderma spp. fungal cultures were placed on the opposite end. These plates were then incubated for seven days at 25 ± 2 °C with three replications, and control plates were also included. As for the bacterial evaluation, 5 mm mycelial discs of the test pathogen were positioned at the center of PDA medium plates. Bacterial cultures were streaked on all four sides of the pathogen disc in a square pattern. Subsequently, these plates were incubated at 28 ± 2 °C for 7 days. Observations were made regarding the radial growth of the test pathogens with or without the presence of the antagonist, and the percentage of inhibition was calculated using the methodology outlined by33. The experiment was replicated for twice.
I is the Per cent inhibition over control. C is the Radial growth of pathogen in control (mm). T is the Radial growth of pathogen in treatment (mm).
Molecular identification of fungal and bacterial biocontrol agents
Based on their observed antagonistic activity, promising bacteria (Eb-8, Eb-11, Eb-13, Eb-21, Rb-4, Rb-11, Rb-14, Rb-18, and Rb-19) were selected and subjected to identification at the species level through 16S rRNA sequencing. Similarly, Trichoderma spp. were identified using TEF1α and ITS region gene sequencing. The CTAB method (Cetyl Trimethyl Ammonium Bromide), was utilized to extract total genomic DNA from both the bacteria and Trichoderma spp. Subsequently, the DNA pellet was dissolved in 50 μL of 1X TAE buffer, which consists of 10 mM Tris and 1 mM EDTA. DNA quantification was carried out on a 0.8% agarose gel, and purity was assessed by determining the A260/A280 ratio using a spectrophotometer. For amplifying the 16S rRNA gene of the bacterial isolates, forward primer (5′-GGATGAGCCHALGGCCTA-3′) and reverse primer (5′-CGGTGTGTACAAGGCCCGG-3′) were used. Subsequently, PCR reactions for Trichoderma spp. were performed using specific primer pairs, namely ITS for amplifying the Internal Transcribed Spacer region of Ribosomal DNA (ITS-rDNA) and Translation Elongation Factor 1-α gene (TEF1α). Eurofins Genomics in Bangalore, Karnataka, sequenced the amplified products using the Sanger sequencing method. Sequences were considered belonging to the same species when they were at least 99.7% identical, and those with at least 97.8% identity were classified as belonging to the same genus.
Characterization and in vitro plant growth promoting activities of bacterial biocontrol agents
Biochemical characterization
A total of nine potential bacterial isolates, known for their antifungal properties against F. udum, underwent thorough biochemical characterization following the guidelines in Bergey's manual of determinative bacteriology. This involved a series of tests, including gram staining, amylase, catalase, oxidase, indole, methyl red, Voges–Proskauer, and citrate utilization tests34.
Plant growth promoting activities
Cellulase production test
The 24 h old bacterial isolates were inoculated on Carboxy Methyl Cellulose (CMC) agar medium plates and incubated at 28 °C for five days to allow the cellulase secretion. Following incubation, the agar medium was soaked in a congo red solution (1 per cent w/v) for 15 min. Subsequently, the congo red solution was drained and the plates were subjected to an additional treatment with 1 M NaCl for 15 min. The presence of a clearly identifiable hydrolysis zone indicated the degradation of cellulose35.
Siderophore production test
CAS (Chrome Azurol S) media was prepared and spot inoculation of the bacterial isolates was done from the actively growing cultures. Colonies that displayed an orange halo zone after 3 days of incubation at 28 ± 2 °C were regarded as positive for siderophore production36.
HCN and ammonia production tests
The method proposed37 was employed to assess the ability of bacteria to produce hydrogen cyanide. Each bacterium was streaked onto a nutrient agar medium containing 4.4 g/L of glycine. A Whatman no. 1 filter paper was placed over the agar, soaked in a specific solution (0.5% picric acid and 2% sodium carbonate w/v). The plates were sealed with parafilm and then incubated for 4 days at 36 ± 2 °C. The presence of an orange or red color indicated the formation of hydrogen cyanide.
The 24 h old bacterial cultures were inoculated in 10 mL of peptone broth and incubated at 28 ± 2 °C for 48–72 h. Later, one mL of Nessler’s reagent was added to each tube and the development of yellow to dark brown colour was taken as a positive reaction. Based on the intensity of colour, the isolates were divided into four groups i.e., + , + + , + + + , + + + + 38.
Phosphate, potassium, and zinc solubilization
The qualitative assessment of phosphate, potassium, and zinc solubilization activities of the isolates was conducted using specific agar media. For phosphate solubilization, pure colonies were spot inoculated onto Pikovskaya’s agar plates and then incubated at 28 ± 2 °C for 5 days. The confirmation of phosphate solubilization was based on the formation of a distinct halo zone around the colony39. Similarly, for potassium solubilization, isolates were spot inoculated onto Aleksandrov agar plates and incubated for 5 days. The presence of a clear halo zone around the colony indicated potassium solubilization40. In the case of zinc solubilization, isolates were spot inoculated onto Tris minimal agar medium supplemented with zinc oxide and then incubated at 30 °C for 3 days. The confirmation of zinc solubilization relied on the formation of a clear halo zone around the colony41. All experiments regarding biochemical tests Plant Growth Promoting Rhizobacteria (PGPR) activities were replicated for validation.
Assessment of selected biocontrol agents against pigeonpea Fusarium wilt under pot conditions
Rhizosphere bacteria (Rb-18) and endophytic bacteria (Eb-21), exhibiting positive antifungal and Plant Growth Promoting Rhizobacteria (PGPR) activities, along with Trichoderma spp. isolated from the Pigeonpea rhizosphere, were selected as biocontrol agents. The experiment utilized seeds of the pigeonpea wilt susceptible cultivar (ICP 2376).
The experimental setup involved pot cultivation using sterilized pots measuring (20 × 15) cm. Each pot was filled with 5 kg of sterilized sandy loamy soil, and 10 surface sterilized seeds were sown for each treatment, with three replications. After 35 days of sowing, five pots were inoculated with a spore suspension of F. udum (50 mL of microconidial suspension containing 1 × 106 conidia/mL per pot). Among these, three pots were inoculated with a Trichoderma spp. spore suspension (6 mL) (1 × 106 spores/mL), and two pots with a bacterial suspension (10 mL of a suspension containing 108 cfu/mL) on the 45th day. Plants that were inoculated with the pathogen and those not treated with either the pathogen or biocontrol agents served as control groups. The greenhouse experiment was conducted under high humidity (≥ 90%) and optimal temperature conditions of 28–30 °C. Each treatment was replicated three times in a completely randomized design.
The per cent disease incidences was calculated by the following formula
Activity of defence enzymes in biocontrol treated plants against Pigeonpea Fusarium wilt
The study evaluated the activity of defense related enzymes, including peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL), in Pigeonpea plants treated with Trichoderma spp. and bacterial biocontrol agents when challenged with F. udum under potted conditions. Fresh leaves were collected randomly from each treatment at different time points: 0, 24, 48, 72 and 96 h after the inoculation with biocontrol agents. The leaf tissues were immersed in liquid nitrogen and homogenized in 10 mL of ice cold 50 mM potassium phosphate buffer (pH 6.8) containing 1 M NaCl, 1 mM EDTA, 1% polyvinyl pyrolidone and 10 mM β-mercaptoethanol. The samples were filtered using muslin cloth and centrifuged at 12,000 rpm at 4 °C for 25 min. The final supernatants were used for the assay of peroxidase and polyphenol oxidase enzymes. The standard assay protocol described by21 was followed for peroxidase and polyphenol oxidase. To determine PAL activity, 400 µL of sample extract was incubated with 0.5 mL of 0.1 M borate buffer pH 8.8 and 0.5 mL of 12 mM l-phenylalanine in the same buffer for 30 min at 30 °C. PAL activity was determined as the rate of conversion of l-phenylalanine to transcinnamic acid at 290 nm. The amount of trans-cinnamic acid synthesised was calculated using its extinction coefficient of 9630 M−1 cm−1. Enzyme activity was expressed in fresh weight basis as nmol trans-cinnamic acid min−1 mg−1 of sample42.
Assessment of selected biocontrol agents against Pigeonpea Fusarium wilt under sick plot conditions
The study was conducted at the AICRP on Pigeonpea wilt sick plot located at T.C. A Dholi, R.P.C.A.U (25° 59′ 41.9″ N 85° 35′ 43.3″ E), Pusa, Bihar. The experiment was carried out over four different seasons, which included Kharif 2021–2022, Rabi 2021–2022, Kharif 2022–2023, and Rabi 2022–2023. To ensure even distribution of the pathogen within the affected plots, four soil samples were taken from each season (3 m × 3 m) plot. These samples underwent a series of dilutions and were then plated on a specialized Fusarium medium following the method outlined by43.
The B. subtilis isolates were inoculated into nutrient broth, while P. aeruginosa isolates were introduced into KB broth. The cultures were then incubated at 28 ± 2 °C 28 ± 2 °C for 36 h on a rotary shaker set at 150 rpm. After incubation, the bacteria were collected through centrifugation at 8000 rpm for 10 m using a benchtop refrigerated centrifuge. The resulting pellets were washed three times with sterile distilled water (SDW) and the cell concentration was adjusted to 1 × 108 colony forming units (cfu) per millilitre through dilution, aiming for suspensions with an optical density of 0.45 at A610 nm, as determined by a UV–visible spectrophotometer (Mortensen, 1992). The Trichoderma spp. isolates were cultured on PDA plates for 10–12 days at 28 ± 2 °C. Subsequently, 10 mL of sterile distilled water (SDW) was added to each plate, and conidia were gently detached from the culture surface by shaking. The remaining conidia were removed using a sterile brush, and the resulting suspension was collected in a 100 mL conical flask. After passing the conidial suspension through four layers of cheesecloth, it was centrifuged at 2500 rpm for 10 min and then resuspended in distilled water. The conidial concentration was adjusted to 1 × 106 conidia per millilitre using a hemocytometer.
Pigeonpea seeds of wilt susceptible cultivar ICP8863 were soaked in a culture suspension with the addition of 0.2% carboxymethyl cellulose (CMC) to aid in the attachment of the biocontrol agent to the seeds. These treated seeds were then incubated at 28 ± 2 °C in a rotary shaker at 150 rpm for 6 h and subsequently air dried under sterile conditions. While carnbendizim was treated as 2.0 mg/g seeds. As a control, seeds soaked in distilled water amended with 0.2% CMC were used. These treated seeds were manually sown in wilt affected plots with a spacing of 90 cm between rows and 20 cm within rows, at a depth of 2–3 cm. The experimental design followed a randomized block pattern with seven treatments, each replicated. Each replication occupied a 3 m × 3 m plot, totalling an area of 9 square meters. The incidence of wilt was assessed 65 days after sowing.
The per cent disease incidences was calculated by the following formula
AMMI analysis
In this study, the performance of seven Treatments (T) and their interactions with four Environments (E) were assessed. Disease incidence data collected from the treatments were organized to be compatible with the AMMI (Additive Main Effects and Multiplicative Interaction) models. The AMMI statistical model, along with computational methods detailed in44, was employed for the analysis. An analysis of variance was conducted to partition the variation into main effects associated with the Treatments (T) and the Environments (E), as well as the interaction effect between Treatments and Environments (T × E). These analyses were carried out using the GEA-R software developed by 'CIMMYT' and the 'R' package Agricolae.
Ethical statement
All authors have approved the manuscript and agreed with its submission to the Scientific Reports. The submitted work is original and has not been submitted or published elsewhere. The manuscript has been prepared following principles of ethical and professional conduct. The study does not involve human participants or animals.
IUCN policy statement
The experimental research and field studies on plants, both cultivated and wild, strictly followed institutional, national, and international guidelines, including the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. Emphasizing our commitment to ethical research, no endangered species of wild fauna and flora were involved, reflecting our dedication to biodiversity conservation and minimizing adverse impacts on vulnerable plant populations. This comprehensive compliance aims to advance scientific knowledge while championing environmental sustainability and global biodiversity preservation, upholding the highest standards of research integrity for the well-being of ecosystems and future generations.
Results
Morphological, pathogenic and molecular characterisation of the pathogen
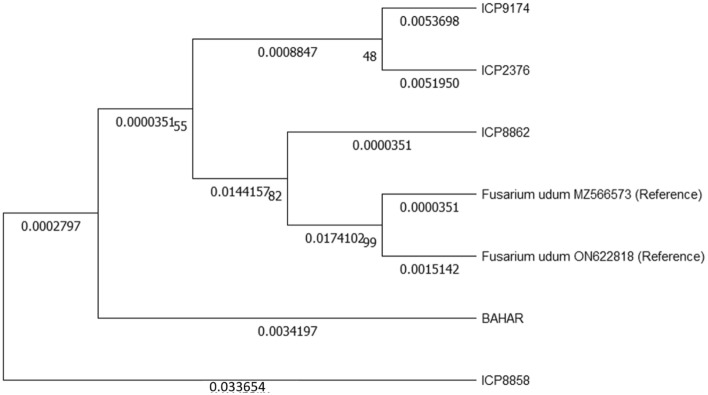
In the present study, a total of five Fusarium isolates were obtained, each originating from a distinct Pigeonpea cultivar (ICP 2376, BAHAR, ICP 8862, ICP 8858, and ICP 9174). The cultural and morphological traits of these Fusarium isolates were investigated on PDA, revealing notable differences in colony texture, substrate pigmentation, mycelial color, and conidia length and width (Supplementary Fig. 3). All Fusarium isolates exhibited pathogenicity in causing wilt disease during the pathogenicity test, with an incidence ranging from 60 to 90%. Notably, the Fusarium isolate obtained from the ICP 8858 cultivar demonstrated the highest disease incidence at 90%, indicating its virulence and was subsequently chosen for further antagonistic investigations. To molecularly characterize these isolates, PCR amplification of the ITS-rDNA region using universal primers yielded amplicons ranging from 500 to 550 bp in length. Additionally, an analysis of nucleotide sequences of the TEF1α gene revealed variations in length, ranging from 670 to 725 base pairs among the five Fusarium isolates. Subsequently, all sequences were submitted to the NCBI GenBank, and accession numbers were obtained for reference and documentation purposes (Table 1) (Fig. 1).
Table 1.
Cultural and morphological characters of F. udum isolates.
| S.no | Cultivar | Radial growth rate (mm) | Mycelium growth pattern | Mycelia colour | Substrate pigmentation | Zonation’s | Macro conidia (µm) | Micro conidia (µm) | Disease incidence (%) | ITS gene accession numbers | TEF gene accession numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ICP 2376 | 86 | Moderately Fluffy | White | Buff | Absent | 24.26 × 2.12 | 8.22 × 3.22 | 60 | OR267399 | PP060448 |
| 2 | BAHAR | 80 | Fluffy | Buff | Buff | Absent | 27.45 × 3.99 | 11.68 × 2.9 | 75 | OR267401 | PP060447 |
| 3 | ICP 8862 | 80 | Appressed | Off White | Yellowish white | Absent | 32.17 × 2.32 | 9.80 × 2.67 | 80 | OR267402 | PP060449 |
| 4 | ICP 8858 | 86 | Fluffy | White | White | Absent | 31.17 × 3.53 | 10.88 × 3.44 | 90 | OR083610 | PP060445 |
| 5 | ICP9174 | 80 | Appressed | Mauve | Plum | Present | 22.17 × 3.52 | 8.88 × 2.65 | 70 | OR267395 | PP060446 |
Figure 1.
Multiple sequence alignment of ITS and TEF genes of Fusarium isolates using Maximum Likelihood method with 1000 boot strap values.
Isolation of beneficial microbes
In our present study, based on cultural and morphological traits a total of 100 endophytic and 100 rhizosphere bacteria were isolated, purified and evaluated for antagonistic activity against F. udum. Simultaneously, we isolated three Trichoderma strains from 10 rhizosphere soil samples and compared them to the Trichoderma Taxonomy database https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=5543 using criteria like conidiospore color and pigment secretion on the PDA medium. Subsequent microscopic examination confirmed the presence of three isolates: T. harzianium, T. asperellum, and an unidentified Trichoderma species. Importantly, two of these isolates, T. harzianium and T. asperellum, were categorized within the Harzianum clade and Hamatum sub branch, respectively, while the third isolate, Trichoderma sp., could not be conclusively identified.
In vitro evaluation of biocontrol agents against F. udum
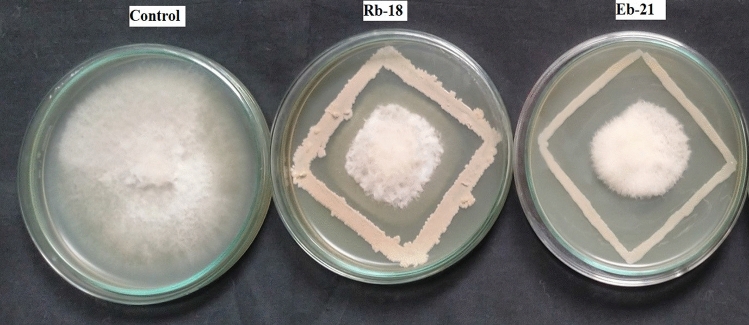
In the dual culture technique, it was noted that among the tested bacterial isolates, four endophytic and five rhizosphere isolates effectively inhibited the growth of F. udum by more than 60%. Specifically, the endophytic bacterial strains identified as Eb-21, Eb-13, Eb-8, and Eb-11 exhibited inhibition percentages of 72.22%, 65.11%, 64.44%, and 62.88%, respectively. In contrast, rhizosphere bacteria labeled as Rb-18, Rb-14, Rb-19, Rb-4, and Rb-11 exhibited inhibition percentages of 71.11%, 68.44%, 65.3%, 64.8%, and 62.11%, respectively (Fig. 2). T. harzianum, T. asperellum, and Trichoderma sp. exhibited inhibition percentages of 65%, 60%, and 55%, respectively, against F. udum.
Figure 2.
Antagonistic activity of bacterial isolates against F. udum: RB-18 (Rhizosphere bacteria) and Eb-21 (Endophytic bacteria).
Molecular based identification of bacterial and fungal isolates
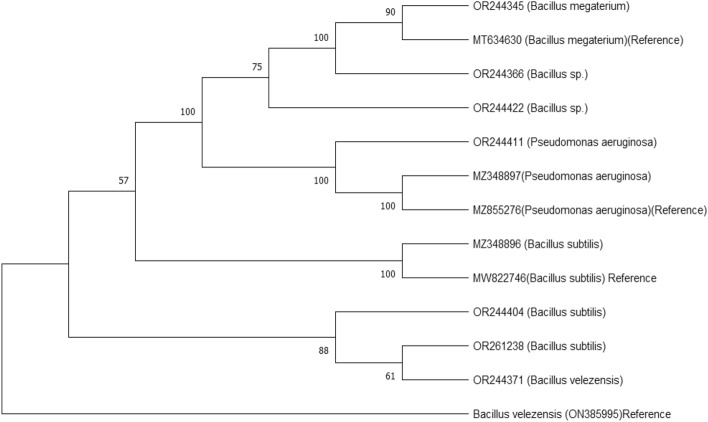
Based on their antifungal characteristics, nine bacterial strains and three Trichoderma species were selected for molecular identification. The Polymerase Chain Reaction (PCR) method was utilized to amplify fragments of the bacterial 16S rRNA gene. Subsequently, the obtained 16S rRNA gene sequences were compared against the NCBI nucleotide database using the Basic Local Alignment Search Tool (BLAST). The results of this comparison led to the identification of the isolates as follows: Rb-4 (Bacillus sp.), Rb-11 (B. subtilis), Rb-14 (B. megaterium), Rb-18 (B. subtilis), Rb-19 (B. velezensis), Eb-8 (Bacillus sp.), Eb-11 (B. subtilis), Eb-13 (P. aeruginosa), and Eb-21 (P. aeruginosa). The genetic sequences were subsequently deposited into the NCBI GenBank, and specific accession numbers were obtained (Fig. 3, Table 2).
Figure 3.
Phylogenetic tree for 16S rRNA gene of bacterial isolates using neighbour-joining method.
Table 2.
Biochemical and molecular characterization of bacterial isolates.
| SI. No | Isolates | Grams reaction | Amylase test | Catalase test | Oxidase test | Indole test | Methyl red test | Voges-Proskauer test | Citrate test | Accession numbers | Organism identified |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eb-8 | + | − | + | − | − | + | − | − | OR244422 | Bacillus sp. |
| 2 | Eb-11 | + | − | + | + | − | − | − | − | OR261238 | B. subtilis |
| 3 | Eb-13 | − | + | + | − | − | + | − | + | OR244411 | P. aeruginosa |
| 4 | Eb-21 | − | + | + | + | − | − | − | − | MZ348897 | P. aeruginosa |
| 5 | Rb-4 | + | + | + | + | − | − | − | + | OR244366 | Bacillus sp. |
| 6 | Rb-11 | + | − | + | + | − | − | − | − | OR244404 | B. subtilis |
| 7 | Rb-14 | + | + | + | + | − | − | − | − | OR244345 | B. megaterium |
| 8 | Rb-18 | + | + | + | + | − | − | − | − | MZ348896 | B. subtilis |
| 9 | Rb-19 | + | + | + | − | − | − | − | − | OR244371 | B. velezensis |
Similarly, for the Trichoderma isolates, BLAST analysis was employed to compare their fungal TEF (Translation Elongation Factor 1-α gene) and small ribosomal gene (18S rRNA gene) sequences with existing Trichoderma sequences in the NCBI database. The BLAST analysis confirmed that the amplified TEF and ITS gene sequences from the Trichoderma isolates showed similarity to known Trichoderma species. Consequently, the sequences were submitted to the NCBI GenBank, securing accession numbers: ITS (MZ348898) TEF (PP060450) for T. harzianum, ITS (MZ411690) TEF (PP060451) for T. asperellum, and ITS (MZ411691) TEF (PP060452) for Trichoderma sp. (Fig. 4).
Figure 4.
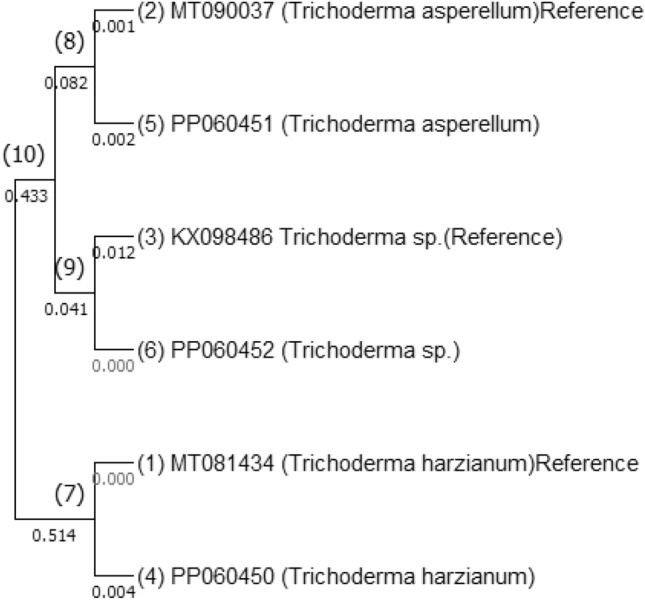
Multiple sequence alignment of ITS and TEF genes of Trichoderma isolates using Maximum Likelihood method with 1000 boot strap values.
Biochemical characterization of bacterial isolates
Bacterial isolates that demonstrated inhibitory effects on F. udum in dual culture experiments underwent biochemical characterization. Among these isolates, all tested positive for the catalase test, seven displayed a positive gram reaction, six exhibited positive results for amylase and oxidase tests and two indicated positive outcomes for citrate utilization and methyl red reduction tests. However, none of the isolates showed a positive result in the indole production test (Table 2).
In vitro plant growth promoting activities
A total of nine potential bacterial isolates, which exhibited inhibitory effects against F. udum in a dual culture technique, underwent in vitro assessment for their growth promoting activities. The cellulase activity of these potential bacterial isolates was evaluated using CMC agar media. The presence of a halo zone around the colony was considered a positive outcome for this test, and variations were observed among the isolates. Specifically, four isolates, namely Eb-8, Eb-21, Rb-14, and Rb-18, exhibited halo zones around their colonies. None of the isolates showed hydrogen cyanide (HCN) production. Interestingly, it was noted that the rhizosphere bacterial population (Rb-18) displayed a higher capacity for siderophore production compared to the endophytic bacteria (Eb-21). Ammonia production was recorded in three isolates Eb-21, Rb-11 and Rb-18.
Additionally, bacterial isolates demonstrating the ability to solubilize inorganic phosphate, potassium, and zinc were assessed based on the formation of clear halo zones in Pikovaskaya’s, Aleksandrov, and Trisminimal agar plates, respectively. In Pikovaskaya’s medium, isolates Eb-21, Rb-14, and Rb-18 exhibited the formation of halo zones (Supplementary Fig. 4). Similarly, on Aleksandrov agar plates, Rb-11 and Rb-18 displayed a halo zone, and on zinc supplemented Trisminimal agar, Eb-21, Rb-11, Rb-14, and Rb-18 exhibited halo zones (Table 3).
Table 3.
In vitro screening of biochemical and enzymes of biocontrol importance: The efficient isolates with biocontrol potential were screened for cellulose, HCN, Siderophores, ammonia production and Phosphorus, Potassium and Zinc solubilisation.
| S. No | Isolate | Cellulase | HCN | Siderophore | Ammonia | Phosphorus | Potassium | Zinc |
|---|---|---|---|---|---|---|---|---|
| 1 | Eb-8 | + | − | − | − | − | − | − |
| 2 | Eb-11 | − | − | − | − | − | − | − |
| 3 | Eb-13 | − | − | − | − | − | − | − |
| 4 | Eb-21 | + + | − | + | + | + | − | + + |
| 5 | Rb-4 | − | − | + + | − | − | − | − |
| 6 | Rb-11 | − | − | + | + | − | + | + |
| 7 | Rb-14 | + + | − | + | − | + | − | + |
| 8 | Rb-18 | + + | − | + + + | + | + + | + | + + |
| 9 | Rb-19 | − | − | − | − | − | − | − |
Assessment of selected biocontrol agents against Pigeonpea Fusarium wilt under pot conditions
The potted plants experiment aimed to evaluate the effectiveness of various biocontrol agents, namely B. subtilis, P. aeruginosa, T. harzianum, T. asperellum, and Trichoderma sp., in reducing Fusarium wilt in Pigeonpea. The disease incidence in the control group without any treatment (T6) was high at 93.33%. However, the treatment involving P. aeruginosa and F. udum (T2) exhibited the lowest disease incidence at 20%. This was followed by the treatments with T. harzianum + F. udum (T3) at 21.66%, B. subtilis + F. udum (T1) at 23.33%, T. asperellum + F. udum (T4) at 26.66%, and Trichoderma sp. + F. udum (T5) at 29.33% (Table 4).
Table 4.
Evaluation of promising biocontrol agents against Pigeonpea Fusarium wilt in pot conditions.
| S.no | Treat no | Treatments | Mean disease incidence |
|---|---|---|---|
| 1 | T1 | B. subtilis + F. udum | 23.33d |
| 2 | T2 | P. aeruginosa + F. udum | 20b |
| 3 | T3 | T. harzianum + F. udum | 21.66c |
| 4 | T4 | T. asperellum + F. udum | 26.66e |
| 5 | T5 | Trichoderma sp. + F. udum | 29.33f |
| 6 | T6 | F. udum | 93.33g |
| 7 | T7 | Control | 0a |
| CD (p = 0.05) 1.512 | |||
Means with same letters between treatments are not significantly different (LSD at p = 0.05).
Activity of defence enzymes in biocontrol treated plants against Pigeonpea Fusarium wilt
In this study, the enzymes associated with plant induced systemic resistance (ISR), including peroxidase (POD), polyphenol oxidase (PPO) and phenylalanine ammonia lyase (PAL), were investigated in vitro. Prospective biocontrol bacteria and Trichoderma spp. isolates were introduced to the plants. The results of the study showed that the highest levels of peroxidase (POD) and polyphenol oxidase (PPO) activity were observed in plants treated with P. aeruginosa + F. udum (1.53) (POD), 1.53 (PPO) and (27) (PAL)) followed by B. subtilis + F. udum and T. harzanium + F. udum. Notably, the POD, PPO, and PAL activity levels were significantly higher in plants treated with bacteria compared to those treated with fungi. Enzyme activity showed a notable increase in all treatments, peaking at 72 h before gradually declining. Control plants, which were neither exposed to the pathogen nor the biocontrol agents, exhibited consistent enzyme activity levels across all time intervals. In contrast, plants treated with the pathogen did not display any significant POD, PPO, or PAL activity when compared to plants treated with the biocontrol agents (Supplementary Figs. 5, 6, 7).
Assessment of selected biocontrol agents against pigeonpea Fusarium wilt under sick plot conditions
The potential fungal and biocontrol agents were applied as seed treatments on the wilt susceptible cultivar ICP2376 and evaluated for their effectiveness against pigeonpea wilt in sick plots over four seasons (2021–2022 Kharif, 2021–2022 Rabi, 2022–2023 Kharif, 2022–2023 Rabi). In all treatments during these four seasons, the lowest mean incidence of the disease was observed in T2 (33.33) (P. aeruginosa) and T3 (35.41) (T. harzanium) followed by T6 (36.5) (Carbendizim), T1 (36.66) (B. subtilis), T4 (52.91) (T. asperellum) and T5 (53.33) (Trichoderma sp.) (Table 5; Fig. 5).
Table 5.
Disease incidence of promising biocontrol agents against Pigeonpea Fusarium wilt in sick plot.
| S.no | Treatments | Kharif 2021–2022 | Rabi 2021–2022 | Kharif 2022–2023 | Rabi 2022–2023 | Mean disease incidence |
|---|---|---|---|---|---|---|
| 1 | B. subtilis + F. udum (T1) | 41.67 | 31.67 | 38.33 | 35 | 36.66a |
| 2 | P. aeruginosa + F. udum (T2) | 33.33 | 30 | 35 | 35 | 33.33a |
| 3 | T. harzanium + F. udum (T3) | 36.67 | 35 | 33.33 | 36.67 | 35.41a |
| 4 | T. asperellum + F. udum (T4) | 53.33 | 53.33 | 51.67 | 53.33 | 52.91b |
| 5 | Trichoderma sp. + F. udum (T5) | 56.67 | 53.33 | 53.33 | 55 | 54.58b |
| 6 | Carbendizim seed treatment (T6) | 36.67 | 33.33 | 38.33 | 37.67 | 36.5a |
| 7 | F. udum (T7) | 91.67 | 95 | 98.33 | 91.67 | 94.16c |
| CD (p = 0.05) 3.728 | ||||||
Means with same letters between treatments are not significantly different (LSD at p = 0.05).
Figure 5.
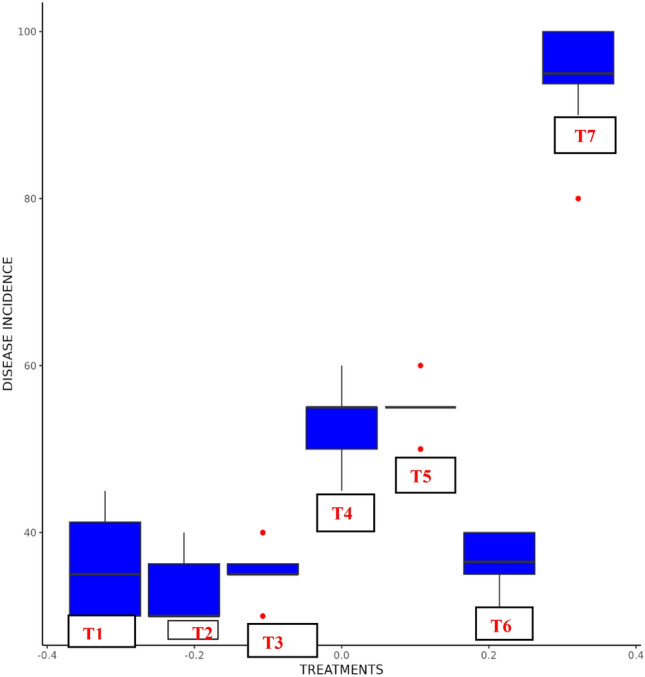
Disease incidence of promising biocontrol agents in Pigeonpea Fusarium wilt sick plot.
AMMI ANNOVA
ANOVA of seven Treatments (T) over four Environments (E) showed that 0.24% of the total SS was attributed to Environments (E) effect; 95.08.% to Treatments (T) effects and 0.88% to Treatments by Environments (T × E) interaction effects. The T × E was further divided into Interaction Principal Component Axis (IPCA) and residuals, in which IPCA1 has contributed (49.01%) of interaction SS followed by IPCA2 which contributed (37.03%) of interaction SS and IPCA1 and IPCA2 cumulatively contributed to (97.411%) of the total interaction (Table 6).
Table 6.
AMMI ANNOVA for biocontrol agent’s treatments × environments interactions.
| S.no | Source | Df | Sum Sq | % Sum of suares | Mean Sq | F value | P |
|---|---|---|---|---|---|---|---|
| 1 | Environment | 3 | 88.41667 | 0.24 | 29.47222 | 0.455169 | S |
| 2 | Treatment | 6 | 33,897.67 | 95 | 5649.611 | 327.5137 | S |
| 4 | Treatment: environment | 18 | 316.3333 | 0.88 | 17.57407 | 1.018787 | S |
| 5 | PC1 | 8 | 154.9392 | 49.01 | 19.3674 | 1.12 | S |
| 6 | PC2 | 6 | 117.1309 | 37.03 | 19.52181 | 1.13 | S |
| 7 | Residuals | 48 | 828 | 2.32 | 17.25 | NA | |
| 8 | Total | 101 | 35,964.75 | 356.0866 | NA |
Df Degree of freedom, SS Sum of squares, MSS Mean sum of squares, IPCA Interaction Principal Components Axis, F F calculated value, P Probability, S Significant.
AMMI 1 Biplot display
The AMMI1 biplot was employed to analyze the average disease incidence and IPCA1 scores of seven treatments in four different environments. It revealed that treatments on the left side of the perpendicular line exhibited lower disease incidence, with T2 having the lowest, followed by T3 and T1. Conversely, treatments on the right side of the perpendicular line displayed higher disease incidence, with T6 having a particularly higher incidence (Fig. 6).
Figure 6.
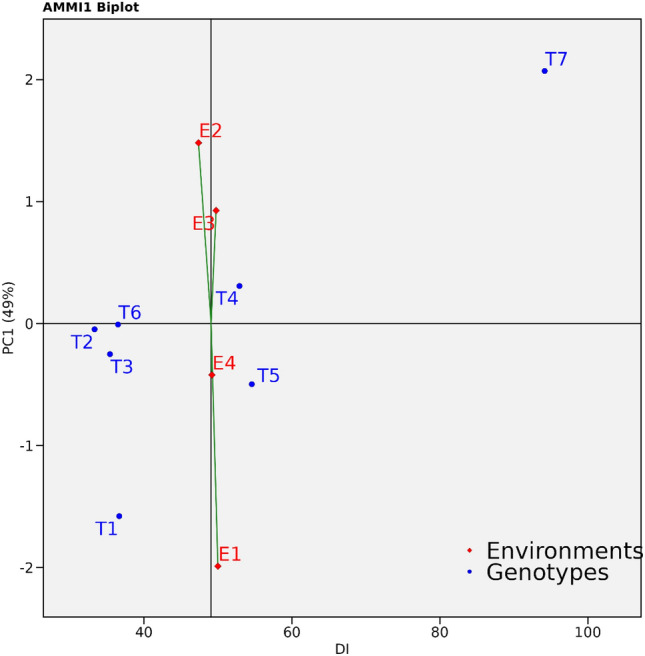
AMMI1 biplot displaying disease incidence and IPCA1 scores of promising biocontrol agents over four environments.
Discussion
Fusarium wilt, caused by the fungus F. udum, poses a significant threat to pigeonpea cultivation worldwide, leading to substantial yield losses3,4. F. udum persists in the soil for extended periods through the formation of chlamydospores and acts as a hemibiotroph when it resides on infected plant remains 2,5. The prolonged persistence of the fungus in the soil and plant debris hampers disease management using conventional methods such as crop rotation and flooding 45,46. Currently, chemical control methods are commonly employed to address this serious wilt disease11. While fungicide application has proven helpful up to seed treatment, it is neither feasible nor economical for crops in the field due to the soil borne nature of F. udum45. Moreover, there is a possibility of the pathogen developing resistance to commonly used fungicides15. Environmental safety concerns also drive the exploration of alternative management strategies that are sustainable in the long run. Although certain resistant pigeonpea cultivars against Fusarium wilt have been identified previously, questions remain regarding the durability of field resistance to F. udum infection over time under field conditions47. Additionally, challenges arise from the evolution of new pathogen variants, the presence of location specific isolates, and the physiological specialization within the Fusarium sp. complex, which hinder successful wilt disease management in pigeonpea. Earlier studies on pathogenic variability in pigeonpea wilt have reported three different pathogenic groups48, five pathogenic variants30, and nine variants7. While soil solarization can address some of these challenges, it has adverse effects on soil quality and beneficial microorganisms49. Biological control emerges as an alternative approach to combat soil borne diseases50.
Biocontrol agents sourced from the native rhizosphere and within plant tissues are preferred due to their adaptability to local soil and climatic conditions51. Moreover, the composition of beneficial microbial populations in the rhizosphere is influenced by both plant root exudates and soil characteristics52. However, the fertile alluvial soils rich in organic matter found in Samastipur and Muzaffarpur districts of Bihar, influenced primarily by sediment deposition from the Gangetic alluvium in the Indo-Gangetic plains, support the growth of bioagents capable of effectively managing wilt diseases and promoting plant growth. Consequently, our recent study aimed to investigate the potential of native microflora isolated from various rhizosphere zones in Bihar for the biocontrol of Fusarium wilt in pigeonpea, as well as for enhancing plant growth. In our study, we assessed 100 endophytic bacteria, 100 native rhizosphere bacteria, and three Trichoderma spp. against F. udum. Among these, four endophytes, five rhizosphere bacteria, and three Trichoderma spp. exhibited inhibition rates exceeding 60% compared to the control, indicating their potential as promising isolates. Similar findings were reported by32,53, who observed that endophytic and rhizosphere bacteria effectively suppressed F. udum growth by inhibiting mycelial development and spore germination. Consistent with our results11 reported that rhizobacteria from pigeonpea demonstrated fungicidal effects against F. udum. This fungicidal activity was attributed to the synthesis of various biocidal substances, including antifungal metabolites, chitinolytic compounds, enzymes capable of breaking down cell walls, and volatile compounds with antifungal properties like ammonia and cyanide.
In laboratory conditions, it was observed that certain rhizobacteria, namely Rb-4, Rb-11, Rb-14, Rb-18, and the endophytic bacterium Eb-21, demonstrated the capability to produce siderophores. In natural soil environments, the production of siderophores is more prevalent among the rhizobacterial community54. The synthesis of siderophores by rhizobacteria plays a crucial role in their capacity to regulate the growth of pathogens. This is achieved by diminishing the availability of ferric ions in the rhizosphere, effectively inhibiting the growth and virulence of soil borne plant pathogens. An illustrative example of this phenomenon is seen in P. aeruginosa, which, when capable of producing siderophores under laboratory conditions, exhibits a broad spectrum of antagonistic effects against pathogens like F. ciceri and F. udum55,56. Similarly, research has indicated that strains of B. atrophaeus and B. subtilis, proficient in siderophore production, can effectively suppress the growth of wilt disease causing pathogens in crops such as cotton (Fusarium oxysporum)57 and pepper58 both under in vitro and in vivo conditions.
Plant Growth Promoting Rhizobacteria (PGPR) possess the ability to produce compounds like hydrogen cyanide (HCN) and ammonia (NH3), which play a dual role in inhibiting fungal growth and promoting plant development59,60. Notably, the ammonia produced by PGPR disperses in the soil, effectively eliminating infectious propagules of specific plant pathogens61. Additionally, it serves as a nitrogen source for host plants, facilitating the growth of roots and shoots, ultimately increasing overall biomass62,63. In our current study, three bacterial isolates, namely Eb-21, Rb-11, and Rb-18, exhibited positive ammonia production. These results align with previous findings on NH3 production by rhizospheric strains of Bacillus and Pseudomonas under in vitro conditions. Furthermore, these strains effectively managed disease incidence caused by F. udum in in vivo conditions11. However, it is important to note that all nine isolates tested negative for the HCN test in this study. In a related investigation by64, it was documented that two rhizosphere strains of B. subtilis and two endophytic bacterium strains of P. aeruginosa also exhibited an inability to produce HCN. Furthermore, biocontrol agents employ critical mechanisms such as cell wall-degrading enzymes, notably cellulase, to regulate soilborne pathogens65. Cellulase exhibits a potent inhibitory effect on the hyphal growth of fungal pathogens by hydrolyzing the 1,4-β-d-glucosidic linkages in cellulose, playing a significant ecological role in recycling cellulose, a major polysaccharide in nature66,67. This degradation process involves various cellulolytic enzymes such as cellulases/endoglucanases, exo-glucanases, and β-glucosidases, which synergistically convert cellulose into β-glucose. In our study, bacterial isolates Eb-8, Eb-21, Rb-14, and Rb-18 exhibited positive cellulase production, consistent with previous findings indicating that biocontrol agents produce lytic enzymes and cellulase to degrade pathogen cell walls68. Similarly, research by11,69 has demonstrated the inhibitory effects of cellulases produced by bacteria from the Bacillus and Pseudomonas genera on the growth of phytopathogenic fungi, thereby contributing to disease suppression in chickpea and pigeonpea wilt.
Phosphorus (P), Potassium (K), and zinc (Zn) are essential macronutrients crucial for biological growth and development. However, the concentrations of soluble P, K, and Zn in the soil are typically low because the majority of these nutrients exist in insoluble forms within rocks, minerals, and other deposits70,71. PGPR play a crucial role in mobilizing these nutrients in the rhizosphere, making them accessible to plants25,72. Under in vitro conditions, rhizosphere bacteria, specifically Rb-18 and Rb-11, demonstrated the ability to solubilize inorganic phosphorus, potassium, and zinc. The solubilization of minerals was notably more efficient in rhizosphere bacteria compared to endophytic bacteria. Several studies have also demonstrated the involvement of rhizospheric Bacillus and Pseudomonas genera in the solubilization of phosphorus, potassium, and zinc under both controlled and field conditions, resulting in enhanced plant growth and yield73–75.
In the potted plant experiment, treatments T2 (P. aeruginosa + F. udum), T3 (T. harzianum + F. udum), T1 (B. subtilis + F. udum), and T4 (T. asperellum + F. udum) demonstrated a significant reduction in the incidence of wilt disease. This aligns with findings from previous studies11,15,76 which also found that native Pseudomonas spp., Bacillus spp., and Trichoderma spp. isolated from the rhizosphere of pigeonpea effectively reduced pigeonpea wilt disease under in vitro experiments.
Beneficial microorganisms often adopt an indirect strategy to enhance a plants resistance against invading phytopathogens by stimulating the plants defense mechanisms. In our study, we focused on inducing Systemic Resistance (ISR) in pigeonpea exposed to antagonistic microbes, including B. subtilis, P. aeruginosa, T. harzianum, T. asperellum, and Trichoderma sp., in the presence of the wilt causing pathogen F. udum. Additionally, we observed that plants inoculated with F. udum but lacking these bioagents exhibited a reduction in the activity of defense related antioxidant enzymes, including POD, PPO, and PAL. The increased activity of the host plant's defense system, particularly the enzymes POD, PPO, and PAL, can be attributed to the secretion of siderophores, chitinase, and protease by these microbes. These compounds act as signaling molecules that activate systemic resistance21. Several studies have demonstrated that Plant Growth Promoting Rhizobacteria (PGPR) can trigger various defense responses in host plant tissues, including the enhancement of antioxidant defense enzyme activity during pathogen attacks77,78. Multiple case studies provide evidence that the inoculation of PGPR can activate ISR related antioxidant enzymes, leading to a reduction in the severity of diseases caused by F. udum in pigeonpea. For instance, treatments involving B. subtilis, P. aeruginosa, and Trichoderma spp. have been shown to activate ISR related antioxidant enzymes, ultimately mitigating the impact of F. udum induced diseases in pigeonpea7.
In subsequent field investigations, the application of seed treatment with antagonistic microbes, including P. aeruginosa (33.33%), T. harzianum (35.41%), B. subtilis (36.66%), and T. asperellum (52.91%), demonstrated effectiveness in reducing the incidence of wilt disease in pigeonpea plants under disease challenged conditions. Numerous rhizosphere microbes have showcased their ability to alleviate the detrimental impacts of both biotic and abiotic stress factors, ultimately fostering plant growth and development79. Previous studies have indicated that T. harzianum and T. asperellum exhibit mycoparasitic activity against soil borne pathogens by releasing compounds such as stigmasterol and ergosterol80,81. Moreover, soil applications of T. harzianum have been demonstrated to reduce the population of F. udum in the soil, consequently decreasing the occurrence of pigeonpea wilt15. Additionally, P. aeruginosa produces antibiotics like oxychlororaphin and phenazine-1-carboxylic acid, which have proven effective in reducing Fusarium wilt in both chickpea and pigeonpea82. Extracellular proteins from B. subtilis have been found to induce flocculation and vacuolation in F. udum mycelium76. The diverse antimicrobial compounds produced by these beneficial microbes hinder the growth, metabolism, and pathogenicity of various fungal phytopathogens52. Consequently, these beneficial fungal and bacterial microbes effectively alleviate the severity of F. udum induced wilt disease. This observation is supported by a report from83 suggesting that antagonistic strains of Pseudomonas, Bacillus, and Trichoderma spp. genera, isolated from the pigeonpea rhizosphere, significantly reduce the severity of wilt disease caused by F. udum in host plants. Additionally, these rhizobacterial inoculations have been shown to enhance the growth characteristics of host plants compared to untreated controls83.
AMMI ANNOVA of all five Treatments (T) over four Environments (E) showed that 0.24% of the total SS was attributed to Environments (E) effect; 95% to Treatments (T) effects and 0.88% to Treatments by Environments (T x E) interaction effects. A large SS for Treatments (T) revealed the huge differences among the mean disease incidence causing most of the variations within the reactions of the treatments84–86.
Conclusion
In summary, this study highlights the serious threat of Fusarium wilt in Pigeonpea and the limited effectiveness of conventional management methods. Indigenous biocontrol agents, such as P. aeruginosa (Eb-21), T. harzianum, and B. subtilis (Rb-18), have shown promise in controlling Fusarium wilt in both lab and field settings. They exhibited antagonistic activity against F. udum, boosted beneficial enzyme activity, and strengthened pigeonpea's resistance mechanisms. Over four seasons of field trials, treatments with P. aeruginosa and T. harzianum consistently had the lowest disease rates. This research emphasizes the potential of these biocontrol agents as sustainable alternatives to traditional fungicides and resistant cultivars for managing Fusarium wilt.
Supplementary Information
Acknowledgements
The authors are grateful to the Departments of Plant Pathology, Departments of Entomology, Department of Agricultural Biotechnology and Molecular Biology of Dr. Rajendra Prasad Central Agricultural University for their unwavering support throughout the research. This research is part of B. Deepak Reddy’s PhD thesis at the RPCAU in Bihar.
Author contributions
B.D.R. Planned and executed the research. B.K., S.S., R.K., U.M. guided the research work. G.Y., and S.K. contributed to Proof reading, DNA extraction, Polymerase chain reaction and analysed the data. M.S.S.R. and K.S.K. helped in manuscript preparation, conducted statistical analysis, and interpretation. All authors approved the final version of the manuscript.
Data availability
The data presented in the study are deposited in the NCBI database (National Center for Biotechnology Information). Accession numbers submitted in NCBI: OR267399 (Fusarium udum), OR267401 (Fusarium udum), OR267402 (Fusarium udum), OR083610 (Fusarium udum), OR267395 (Fusarium udum), OR244422 (Bacillus sp.), OR261238 (B. subtilis), OR244411 (P. aeruginosa), MZ348897 (P. aeruginosa), OR244366 (Bacillus sp), OR244404 (B. subtilis), OR244345 (B. megaterium), MZ348896 (B. subtilis), OR244371 (B. velezensis), MZ348898 (T. harzianium) MZ411690 (T. asperellum) and MZ411691 (Trichoderma sp.).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60039-0.
References
- 1.Behera SK, Shukla AK, Tiwari PK, Tripathi A, Singh P, Trivedi V, Patra AK, Das S. Classification of Pigeonpea (Cajanus cajan (L.) Millsp.) genotypes for zinc efficiency. Plants. 2020;9:952. doi: 10.3390/plants9080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy BD, Kumar B, Reddy MSS, Krishna SK, Kumar R, Karthik S. Identification of elite pigeon pea genotypes against Fusarium Wilt and Sterility Mosaic Disease through AMMI and GGE biplot Analyses. Legume Res. 2023;46:104–111. doi: 10.18805/LR-4838. [DOI] [Google Scholar]
- 3.Sharma M, Ghosh R, Telangre R, Rathore A, Saifulla M, Mahalinga DM, Saxena DR, Jain YK. Environmental influences on pigeonpea-Fusarium udum interactions and stability of genotypes to Fusarium wilt. Front. Plant Sci. 2016;7:e.253. doi: 10.3389/fpls.2016.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava AK, Srivastava R, Yadav J, Singh AK, Tiwari PK, Srivastava AK, Sahu PK, Singh SM, Kashyap PL. Virulence and pathogenicity determinants in whole genome sequence of Fusarium udum causing wilt of pigeonpea. Front. Microbiol. 2023;14:271. doi: 10.3389/fmicb.2023.1066096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nene YL, Kannaiyan J, Reddy MV, Zote KK, Mahmood M, Hiremath RV, Shukla P, Kotasthane SR, Sengupta K, Jha PK, Haque MF, Grewal JS. Multilocational testing of pigeonpea for broad based resistance to Fusarium wilt resistance in India. Indian Phytopath. 1989;42:449–453. [Google Scholar]
- 6.Reddy MV, Nene YL, Kanhaiya J, Raju TN, Saka VN, Davor AT, Sunga WP, Manga P. Pigeonpea lines resistant to wilt in Kenya and Malawi. Int. Pigeonpea Newsl. 1990;6:34. [Google Scholar]
- 7.Ravikumara BM, Ramangowda G, Naik KM, Telangre R, Sharma M. Distribution and pathogenic diversity in Fusarium udum Butler isolates: The causal agent of pigeonpea Fusarium wilt. BMC Plant Biol. 2022;22:1–12. doi: 10.1186/s12870-022-03526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay RS, Rai B. A new disease cycle of wilt of pigeon-pea. Curr. Sci. 1983;52:978–981. [Google Scholar]
- 9.Manikandan A, Jaivel N, Johnson I, Krishnamoorthy R, Senthilkumar M, Raghu R, Gopal NO, Mukherjee PK, Anandham R. Suppression of Macrophomina root rot, Fusarium wilt and growth promotion of some pulses by antagonistic rhizobacteria. Physiol. Mol. Plant Pathol. 2022;121:101876. doi: 10.1016/j.pmpp.2022.101876. [DOI] [Google Scholar]
- 10.Singh SK, Singh RH, Dutta S. Integrated management of pigeonpea wilt by biotic agents and biopesticides. Ann. Plant Prot. Sci. 2002;10:323–326. [Google Scholar]
- 11.Dukare A, Paul S. Biological control of Fusarium wilt and growth promotion in pigeon pea (Cajanus cajan) by antagonistic rhizobacteria, displaying multiple modes of pathogen inhibition. Rhizosphere. 2021;17:100278. doi: 10.1016/j.rhisph.2020.100278. [DOI] [Google Scholar]
- 12.Li Y, Sun R, Yu J, Saravanakumar K, Chen J. Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 2016;56:318–327. doi: 10.1007/s12088-016-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay R, Kumar D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control. 2020;2020(30):1–8. doi: 10.1186/s41938-020-00333-x. [DOI] [Google Scholar]
- 14.Yassin MT, Mostafa AAF, Al-Askar AA, Sayed SR, Rady AM. Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud Univ. Sci. 2021;33:101363. doi: 10.1016/j.jksus.2021.101363. [DOI] [Google Scholar]
- 15.Prasad RD, Rangeshwaran R, Hegde SV, Anuroop C. Effect of soil and seed application of Trichoderma harzianum on pigeonpea wilt caused by Fusarium udum under field conditions. Crop Prot. 2002;21:293–297. doi: 10.1016/S0261-2194(01)00100-4. [DOI] [Google Scholar]
- 16.Mahapatra S, Yadav R, Ramakrishna W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022;132:3543–3562. doi: 10.1111/jam.15480. [DOI] [PubMed] [Google Scholar]
- 17.Sivasakthi S, Usharani G, Saranraj P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014;9(16):1265–1277. [Google Scholar]
- 18.Wei Z, Gu Y, Friman VP, Kowalchuk GA, Xu Y, Shen Q. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019;5:0759. doi: 10.1126/sciadv.aaw0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake C, Christensen MN, Kovács ÁT. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 2021;34:15–25. doi: 10.1094/MPMI-08-20-0225-CR. [DOI] [PubMed] [Google Scholar]
- 20.Singh SK, Pathak R, Choudhary V. Plant growth-promoting rhizobacteria-mediated acquired systemic resistance in plants against pests and diseases. Microb. Mediat. Induced Syst. Resist. Plants. 2016 doi: 10.1007/978-981-10-0388-2_8. [DOI] [Google Scholar]
- 21.Rais A, Jabeen Z, Shair F, Hafeez FY, Hassan MN. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS One. 2017;12:0187412. doi: 10.1371/journal.pone.0187412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 23.Bowling AC, Crowden RK. Peroxidase activity and lignification in the pod membrane of Pisum Sativum L. Aust. J. Biol. Sci. 1973;26:679–684. doi: 10.1071/BI9730679. [DOI] [Google Scholar]
- 24.Reuveni R, Shimoni M, Crute IR. An association between high peroxidase activity in lettuce (Lactuca sativa) and field resistance to downy mildew (Bremia lactucae) J. Phytopathol. 1991;132:312–318. doi: 10.1111/j.1439-0434.1991.tb00126.x. [DOI] [Google Scholar]
- 25.Wang J, Li R, Zhang H, Wei G, Li Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020;20:1–12. doi: 10.1186/s12866-020-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta S, Mishra AK, Kumar BD. Induction of systemic resistance against fusarial wilt in Pigeonpea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol. Biochem. 2008;40:452–461. doi: 10.1016/j.soilbio.2007.09.009. [DOI] [Google Scholar]
- 27.Bello OA, Esan EB, Obembe OO. Establishing surface sterilization protocol for nodal culture of Solanecio biafrae. IOP Conf. Ser. Earth Environ. Sci. 2018;210:012007. doi: 10.1088/1755-1315/210/1/012007. [DOI] [Google Scholar]
- 28.Booth, C. The genus Fusarium, vol. 114 (Common Wealth Mycological Institute, 1971).
- 29.Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. Am. Phytopthol. Soc. 1998;4:130. [Google Scholar]
- 30.Dhar V, Datta S, Chaudhary RG, Upadhyay JP, Saifulla M, Mishra S, Prajapti RK, Shamim M. Pathogenic and molecular characterisations of pigeonpea wilt pathogen Fusarium udum. Arch. Phytopathol. Plant Prot. 2012;45:423–436. doi: 10.1080/03235408.2011.587974. [DOI] [Google Scholar]
- 31.Vlassak K, Holm VL, Duchateau L, Vanderleyden J, De Mot R. Isolation and characterization of fluorescent Pseudomonas associated with the roots of rice and banana grown in Sri Lanka. Plant Soil. 1992;145:51–63. doi: 10.1007/BF00009541. [DOI] [Google Scholar]
- 32.Swagatika, P. Studies on biological management of wilt Disease (Fusarium udum Butler) in redgram [Cajanus cajan (L.) Millsp.]. (Masters dissertation, Professor Jayashankar Telangana State Agricultural University, 2019.
- 33.Vincent JM. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;159:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]
- 34.MacFaddin JF. Biochemical Tests for Identification of Medical Bacteria. 3. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 35.Ariffin H, Abdullah N, Umi KMS, Shirai Y, Hassan MA. Production and characterization of cellulase by Bacillus pumilus EB3. Int. J. Eng. Technol. 2006;3:47–53. [Google Scholar]
- 36.Louden BC, Harrman D, Lynne MA. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011;12:51–53. doi: 10.1128/jmbe.v12i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorck H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948;1:142–146. doi: 10.1111/j.1399-3054.1948.tb07118.x. [DOI] [Google Scholar]
- 38.Cappuccino JG, Sherman N. Microbiology: A Laboratory Manual. The Benjamin/Comings Publishing Company, Inc.; 1992. [Google Scholar]
- 39.Pikovskaya RI. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 40.Prajapati KB, Modi HA. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech J. Microbiol. 2012;1:8–14. [Google Scholar]
- 41.Saravanan VS, Subramoniam SR, Raj SA. Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Braz. J. Microbiol. 2004;35:121–125. doi: 10.1590/S1517-83822004000100020. [DOI] [Google Scholar]
- 42.Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984;25:111–123. doi: 10.1016/0048-4059(84)90050-X. [DOI] [Google Scholar]
- 43.Nash Shirley M, Snyder WC. Quantitative estimations by plate counts of propagules of the bean root rot Fusarium in field soils. Phytopathology. 1962;52:567–572. [Google Scholar]
- 44.Gauch HG. A simple protocol for AMMI analysis of yield trials. Crop Sci. 2013;53:1860–1869. doi: 10.2135/cropsci2013.04.0241. [DOI] [Google Scholar]
- 45.Eljounaidi K, Lee SK, Bae H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases review and future prospects. Biol. Control. 2016;103:62–68. doi: 10.1016/j.biocontrol.2016.07.013. [DOI] [Google Scholar]
- 46.Dusengemungu, L. Fusarium Soilborne Pathogen. In Fusarium-An Overview of the Genus (Intech Open, 2021) 10.5772/intechopen.100597.
- 47.Mishra RK, Mishra M, Naik SS, Bohra A, Naimuddin K, Dutta D, Kumar K, Singh F, Singh IP. Identification of resistance sources against Fusarium udum (Race-2) in wild accessions of Pigeonpea for strengthening the pre-breeding program. Indian Phytopathol. 2022;75:1–7. doi: 10.1007/s42360-022-00560-2. [DOI] [Google Scholar]
- 48.Reddy, M. V., Raju, T. N., Lenne, J. M. Diseases of pigeonpea. In The Pathology of Food and Pasture Legumes (eds. Allen, D. J., Lenne, J. M.) 517–558 (CAB International, 1999).
- 49.Gill HK, Aujla IS, De Bellis L, Luvisi A. The role of soil solarization in India: How an unnoticed practice could support pest control. Front. Plant Sci. 2017;8:278908. doi: 10.3389/fpls.2017.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Wu G, Deng R, Hu X, Tan H, Chen Y, Tian Z, Li J. Spatiotemporal biocontrol and rhizosphere microbiome analysis of Fusarium wilt of banana. Commun. Biol. 2023;6:27. doi: 10.1038/s42003-023-04417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Li J, Zhang Z, Liu Y, Wei Y. Tomato endophytic bacteria composition and mechanism of suppressiveness of wilt disease (Fusarium oxysporum) Front. Microbiol. 2021;12:731764. doi: 10.3389/fmicb.2021.731764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berini F, Katz C, Gruzdev N, Casartelli M, Tettamanti G, Marinelli F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018;36:818–838. doi: 10.1016/j.biotechadv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Kong W, Li P, Wu X, Wu T, Sun X. Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms. 2020;8:590. doi: 10.3390/microorganisms8040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front. Microbiol. 2016;6:1360. doi: 10.3389/fmicb.2015.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sulochana, M. B., Jayachandra, S. Y., Kumar, S. A., Parameshwar, A. B, Reddy, K. M., Dayanand, A. Siderophore as a potential plant growth-promoting agent produced by Pseudomonas aeruginosa JAS-25. Appl. Biochem. Biotechnol. 174, 297–308. 10.1007/s12010-014-1039-3 (2014). [DOI] [PubMed]
- 56.Dave A, Ingle S. Streptomyces sp. S-9 promotes plant growth and confers resistance in Pigeon pea (Cajanus cajan) against Fusarium wilt. 3 Biotech. 2021;11:459. doi: 10.1007/s13205-021-02989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin TX, Xu CH, Tang M, Guan QL, Gong MF. Siderophore producing by endophytic bacterial strain YBS106 with antifungal activity against Fusarium oxysporum. J. Pure Appl. Microbiol. 2013;7:2091–2096. [Google Scholar]
- 58.Yu X, Ai C, Xin L, Zhou G. The siderophore producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011;47:138–145. doi: 10.1016/j.ejsobi.2010.11.001. [DOI] [Google Scholar]
- 59.Kumar A, Kumar A, Devi S, Patil S, Payal C, Negi S. Isolation, screening and characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities: An in vitro study. Recent Res. Sci. Technol. 2012;4:1–5. [Google Scholar]
- 60.Richard PO, Adekanmbi AO, Ogunjobi AA. Screening of bacteria isolated from the rhizosphere of maize plant (Zea mays L.) for ammonia production and nitrogen fixation. Afr. J. Microbiol. Res. 2018;12:829–834. doi: 10.5897/AJMR2018.8957. [DOI] [Google Scholar]
- 61.Mota MS, Gomes CB, Souza IT, Moura AB. Bacterial selection for biological control of plant disease: Criterion determination and validation. Braz. J. Microbiol. 2017;48:62–70. doi: 10.1016/j.bjm.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhattacharyya C, Banerjee S, Acharya U, Mitra A, Mallick I, Haldar A, Haldar S, Ghosh A, Ghosh A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020;10:15536. doi: 10.1038/s41598-020-72439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdel-Hamid MS, Fouda A, El-Ela HKA, El-Ghamry AA, Hassan SED. Plant growth-promoting properties of bacterial endophytes isolated from roots of Thymus vulgaris L. and investigate their role as biofertilizers to enhance the essential oil contents. Biomol. Concepts. 2021;12:175–196. doi: 10.1515/bmc-2021-0019. [DOI] [PubMed] [Google Scholar]
- 64.Reetha AK, Pavani SL, Mohan S. Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina phaseolina (Tassi.) Goid. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:172–178. [Google Scholar]
- 65.Sadfi N, Cherif M, Fliss I, Boudabbous A, Antoun H. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J. Plant Pathol. 2001;83:101–111. [Google Scholar]
- 66.Jadhav, H. P., Shaikh, S. S., Sayyed, R. Z. Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: An overview. In Rhizotrophs: Plant Growth Promotion to Bioremediation. 183–203. 10.1007/978-981-10-4862-3_9 (2017).
- 67.Saeed Q, Xiukang W, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M, Brtnicky M. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021;22:10529. doi: 10.3390/ijms221910529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalifa M, Rouag N, Bouhadida M. Evaluation of the antagonistic effect of Pseudomonas rhizobacteria on Fusarium wilt of chickpea. Agriculture. 2022;12:429. doi: 10.3390/agriculture12030429. [DOI] [Google Scholar]
- 69.Bhargavi G, Arya M, Jambhulkar PP, Singh A, Rout AK, Behera BK, Chaturvedi SK, Singh AK. Evaluation of biocontrol efficacy of rhizosphere dwelling bacteria for management of Fusarium wilt and Botrytis gray mold of chickpea. BMC Genom. Data. 2024;25:7. doi: 10.1186/s12863-023-01178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldstein AH. Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by gramnegative bacteria. In: Torriani-Gorni A, Yagil E, Silver S, editors. Phosphate in Microorganisms: Cellular and Molecular Biology. ASM Press; 1994. pp. 197–203. [Google Scholar]
- 71.Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK, Suman A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann. Microbiol. 2015;65:1885–1899. doi: 10.1007/s13213-014-1027-4. [DOI] [Google Scholar]
- 72.Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- 73.Devi SP, Ranjithkumar R, Djearamane S, Tey LH, Wong LS, Kayarohanam S, Arumugam N, Almansour AI, Perumal K. Organic remobilization of zinc and phosphorus availability to plants by application of mineral solubilizing bacteria Pseudomonas aeruginosa. Heliyon. 2023;9:e22128. doi: 10.1016/j.heliyon.2023.e22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srithaworn M, Jaroenthanyakorn J, Tangjitjaroenkun J, Suriyachadkun C, Chunhachart O. Zinc solubilizing bacteria and their potential as bioinoculant for growth promotion of green soybean (Glycine max L. Merr.) PeerJ. 2023;11:e15128. doi: 10.7717/peerj.15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iftikhar A, Farooq R, Akhtar M, Khalid H, Hussain N, Ali Q, Malook SU, Ali D. Ecological and sustainable implications of phosphorous-solubilizing microorganisms in soil. Discov. Appl. Sci. 2024;6:33. doi: 10.1007/s42452-024-05683-x. [DOI] [Google Scholar]
- 76.Harish S, Manjula K, Podile AR. Fusarium udum is resistant to the mycolytic activity of a biocontrol strain of Bacillus subtilis AF 1. FEMS Microbiol. Ecol. 1998;25:385–390. doi: 10.1111/j.1574-6941.1998.tb00490.x. [DOI] [Google Scholar]
- 77.Senthilraja G, Anand T, Kennedy JS, Raguchander T, Samiyappan R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leaf miner insect and collar rot pathogen. Physiol. Mol. Plant Pathol. 2013;82:10–19. doi: 10.1016/j.pmpp.2012.12.002. [DOI] [Google Scholar]
- 78.Bano A, Muqarab RJPB. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.) Plant Biol. 2017;19:406–412. doi: 10.1111/plb.12535. [DOI] [PubMed] [Google Scholar]
- 79.Sangwan, S., Dukare, A. Microbe-mediated bioremediation: an eco-friendly sustainable approach for environmental clean-up. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Soil-Microbe Interaction. 145–163. 10.1007/978-981-10-6178-3_8 (2018).
- 80.Chowdhary K, Sharma S. Plant growth promotion and biocontrol potential of fungal endophytes in the inflorescence of Aloe vera L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020;90:1045–1055. doi: 10.1007/s40011-020-01173-3. [DOI] [Google Scholar]
- 81.Win TT, Bo B, Malec P, Khan S, Fu P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021;103:549–561. doi: 10.1007/s42161-021-00780-x. [DOI] [Google Scholar]
- 82.Anjaiah V, Cornelis P, Koedam N. Effect of genotype and root colonization in biological control of Fusarium wilts in pigeonpea and chickpea by Pseudomonas aeruginosa PNA1. Can. J. Microbiol. 2003;49:85–91. doi: 10.1139/w03-011. [DOI] [PubMed] [Google Scholar]
- 83.Karimi E, Safaie N, Shams-Baksh M, Mahmoudi B. Bacillus amyloliquefaciens SB14 from rhizosphere alleviates Rhizoctonia damping-off disease on sugar beet. Microbiol. Res. 2016;192:221–230. doi: 10.1016/j.micres.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Nayak D, Bose LK, Singh S, Nayak P. Additive main effects and multiplicative interaction analysis of host pathogen relationship in rice bacterial blight pathosystem. Plant Pathol. J. 2008;24:337–351. doi: 10.5423/PPJ.2008.24.3.337. [DOI] [Google Scholar]
- 85.Persaud R, Saravanakumar D, Persaud M. Identification of resistant cultivars for sheath blight and use of AMMI models to understand genotype and environment interactions. Plant Dis. 2019;103:2204–2221. doi: 10.1094/pdis-12-18-2301-re. [DOI] [PubMed] [Google Scholar]
- 86.Deepak Reddy B, Kumar B, Sahni S, Yashaswini G, Karthik S, Reddy MSS, Kumar R, Mukherjee U. Genetic diversity and population structure of Fusarium udum in India and its correlation with pigeonpea wilt incidence. J. Basic Microbiol. 2024;64(1–16):2024. doi: 10.1002/jobm.202300682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the study are deposited in the NCBI database (National Center for Biotechnology Information). Accession numbers submitted in NCBI: OR267399 (Fusarium udum), OR267401 (Fusarium udum), OR267402 (Fusarium udum), OR083610 (Fusarium udum), OR267395 (Fusarium udum), OR244422 (Bacillus sp.), OR261238 (B. subtilis), OR244411 (P. aeruginosa), MZ348897 (P. aeruginosa), OR244366 (Bacillus sp), OR244404 (B. subtilis), OR244345 (B. megaterium), MZ348896 (B. subtilis), OR244371 (B. velezensis), MZ348898 (T. harzianium) MZ411690 (T. asperellum) and MZ411691 (Trichoderma sp.).