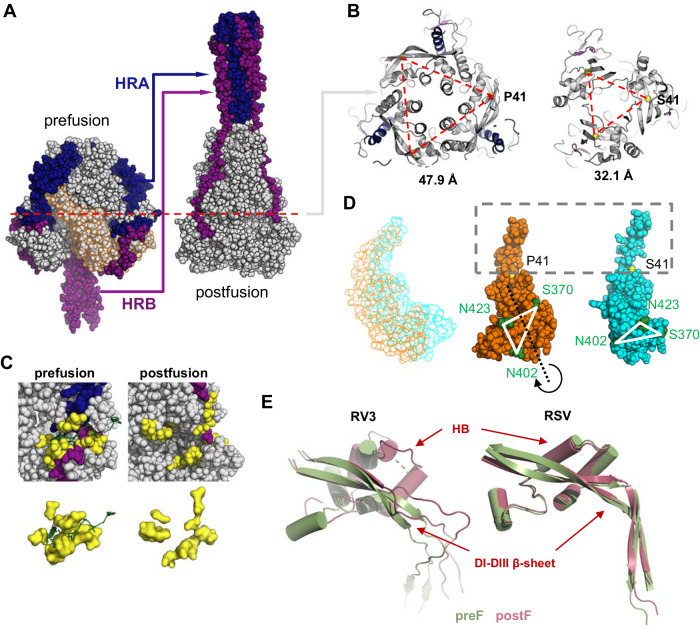
Fig. 8. Structural transformation of the RV3 preF conformation.
A The prefusion structure (OnlyEcto2P) compared with the postfusion structure of RV3 F (PDB ID 1ZTM65). HRA and HRB, including the preceding loop, are colored in blue and purple respectively. DI and DII in the prefusion structure are indicated with an orange outline. B Cross-section indicated in (A) for the prefusion (left) and postfusion (right) structures. C-alpha of residue 41 has been indicated as yellow sphere at the corner of the triangle and the distances between the C-alpha’s of the three monomers are indicated below. C Fusion peptide (green) pocket (yellow) for the preF and postF structures were plotted in the context of the full protein (upper panels) and without it (lower panels). D left panel Translation of DI and DII during the conformational change, with the preF structure indicated in orange and postF in cyan. Middle and right panel Aligned on the beta sheet around residue 41, with three residues in green to indicate the rotation of the domain during the refolding. E Comparison of the conformational change of the DI-DIII β-sheet structure and helical bundle (HB) between RV3 F and RSV F, with the preF structure indicated in green and postF in red. The structures were aligned based on the top part of the DI-DIII β-sheet.