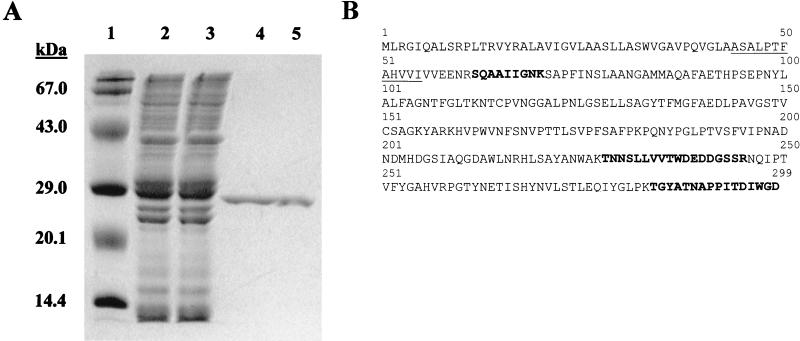
FIG. 1.
Purification of acid phosphatase activity from culture filtrate of M. tuberculosis. (A) Coomassie blue-stained SDS-polyacrylamide gel (12% polyacrylamide) showing the purification of the M. tuberculosis acid phosphatase. Lane 1, molecular mass markers; lane 2, M. tuberculosis CFPs; lane 3, nonbinding proteins from cation-exchange chromatography; lane 4, a pool of fractions 12 to 30 from cation-exchange chromatography; lane 5, purified SapM after hydrophobic-interaction chromatography. (B) Amino acid sequence of the Rv3310 gene product (SapM). The solid line indicates the N-terminal amino acid sequence obtained from the mature protein. The bold sequences indicate individual peptides identified by MS-MS analysis of a tryptic digest of purified SapM. The boxes indicate His residues conserved in fungal acid phosphatase homologues.