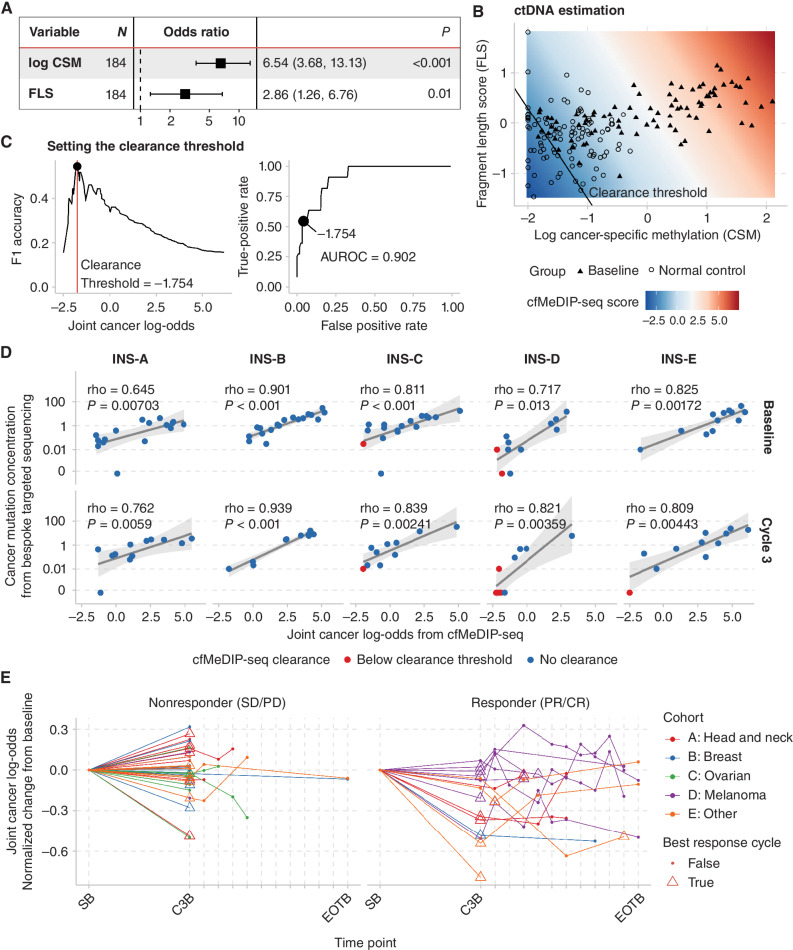
Figure 2.
Joint analysis of cell-free methylomes and fragmentomes enables accurate estimation of ctDNA abundance. A, We fit a logistic regression model with cancer vs. noncancer as the response variable using cfMeDIP-seq data from 85 blood samples from patients with baseline advanced cancer and 100 normal controls. CSM and FLS were each independently associated with cancer. B, The predictions of the logistic regression model can be interpreted as a joint cfMeDIP-seq score corresponding to the log odds of a sample arising from a patient with cancer. C, Using this score, we found that a threshold of −1.754 best identified cases which had undetectable ctDNA based on targeted deep sequencing of cancer-specific mutations. D, cfMeDIP-seq scores correlated with tumor-informed CMC determined by tumor-informed bespoke array (SignateraTM) in all cohorts at baseline (SB) and cycle 3 (C3B). E, cfMeDIP-seq scores vary dynamically across time points and decrease more in patients who exhibit PR or CR to pembrolizumab.