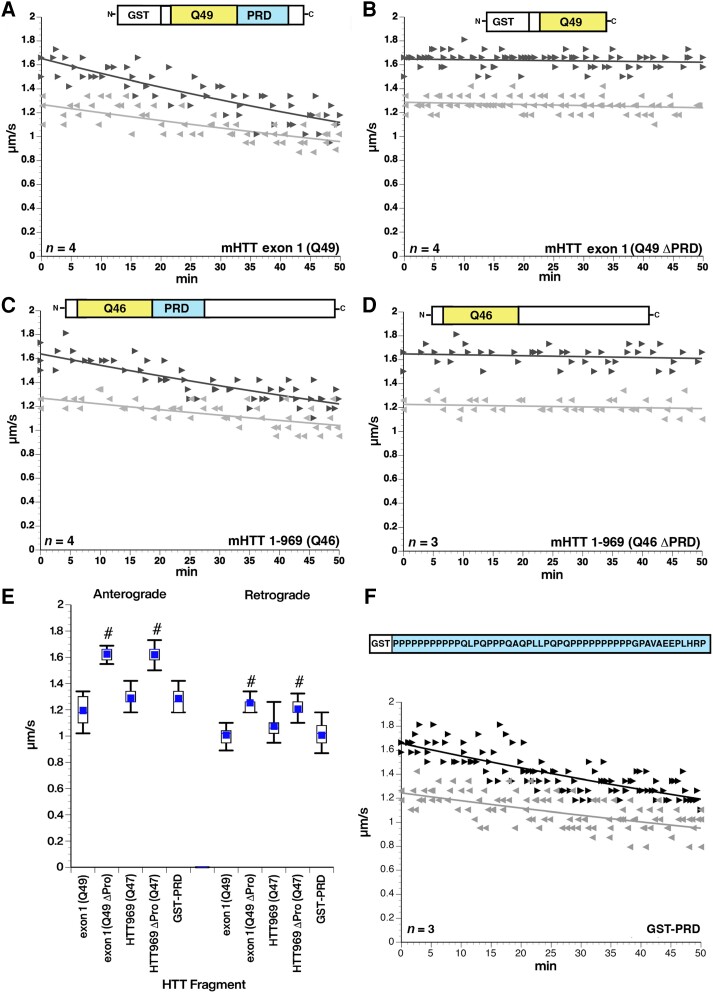
Figure 2.
Deletion of the PRD eliminate toxic effects of mHTT fragments on fast axonal transport. (A) Perfusion of mHTT exon 1 (Q49) at 100 nM inhibits fast axonal transport in both directions through activation of the MAPK JNK3,35 (B) The same mHTT construct with the proline-rich domain (PRD) deleted [mHTT exon 1 (Q49 ΔPRD)] has no effect on axonal transport in either direction at 100 nM. (C and D) Similar effects are seen with a longer mHTT (1–969 Q46) fragment at 100 nM. Deletion of the PRD [mHTT (1–969 Q46 ΔPRD)] eliminates the toxic effects on fast axonal transport elicited by mHTT (1–969 Q46). (E) Quantitative comparison of rates observed between 30 and 50 min with each construct shows that the PRD is necessary for the toxicity of both mHTT exon 1 (Q49) and mHTT (1–969 Q46). #P < 0.0001. (F) A glutathione-S-transferase (GST) tagged protein in which the PRD from HTT is added to the C-terminus (GST-PRD) has the same effect on both anterograde and retrograde axonal transport without the presence of a polyQ stretch. The inhibitory effect of GST-PRD is not significantly different from the effects of pathogenic forms of HTT with an expanded polyQ. This suggests that the PRD is both necessary and sufficient to produce the effect of pathological mutant HTT on fast axonal transport. n = number of axoplasms.