Figure 2: Tumour cell mechanosensing, mechanotransduction, and mechanical memory of matrix stiffness imprinted through persistent epigenetic changes.
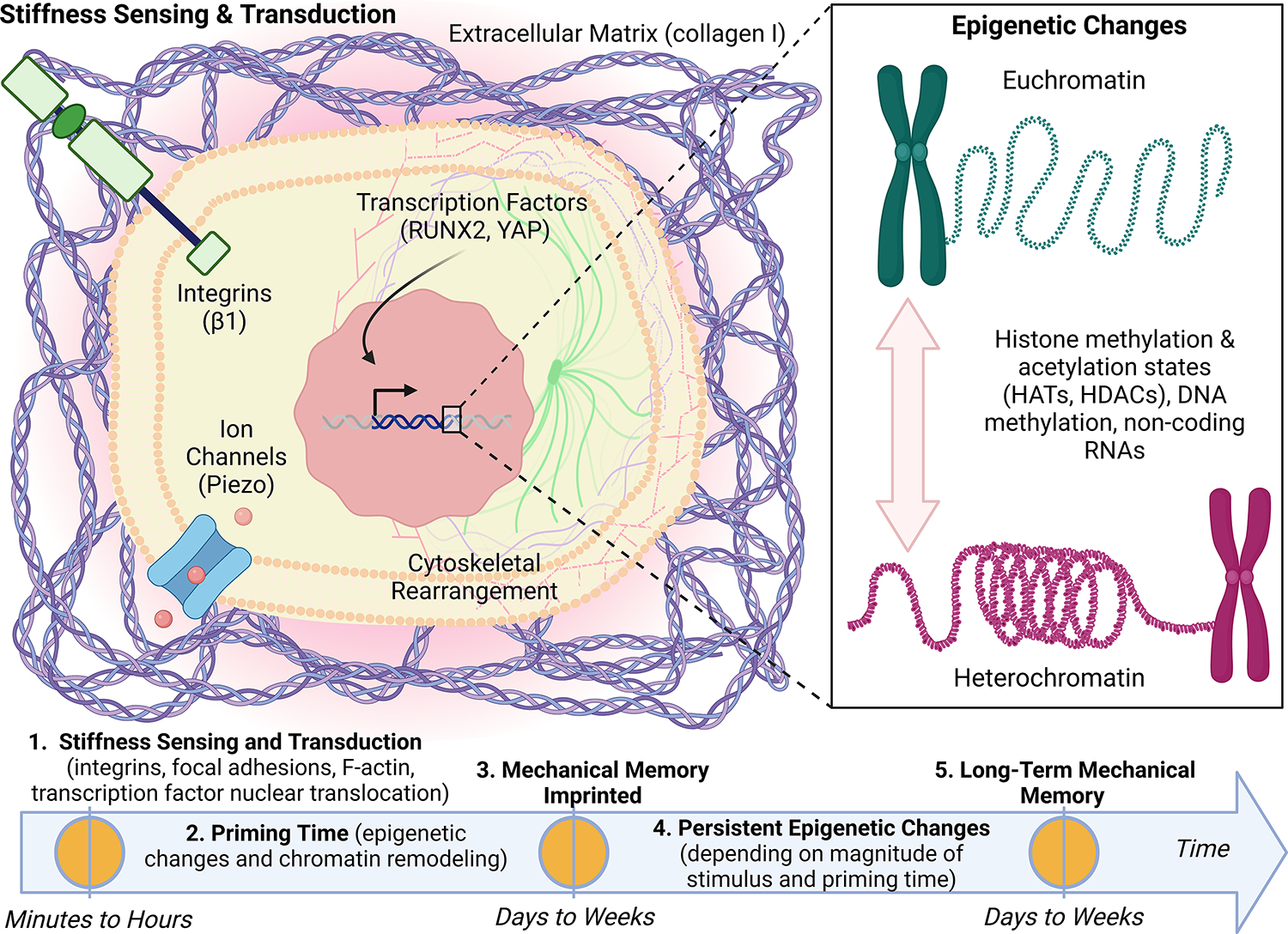
Tumour cells sense high matrix stiffness via the activation of integrins which promotes cytoskeletal rearrangement that in turn promote nuclear translocation of mechanotransducive transcription factors, runt-related transcription factor 2 (RUNX2) and yes-associated protein (YAP). These mechanosensing and mechanotransduction mechanisms occur within minutes to hours of exposure to stiff matrix. On a longer timescale, mechanotransduction can lead to epigenetic changes including histone modification through histone acetyltransferases (HATs) and histone deacetylases (HDACs), DNA methylation, and non-coding RNAs. If the magnitude of the mechanical stress and priming time are sufficient (days to weeks), we hypothesize that the phenotypic adaptations will be maintained via tumour cell mechanical memory encoded by persistent epigenetic changes. We propose that mechanical memory mechanisms play a fundamental role in the metastatic process by directly linking tumour cell biophysical adaptations acquired in the primary tumour to the adaptations that drive disease progression in secondary sites.
