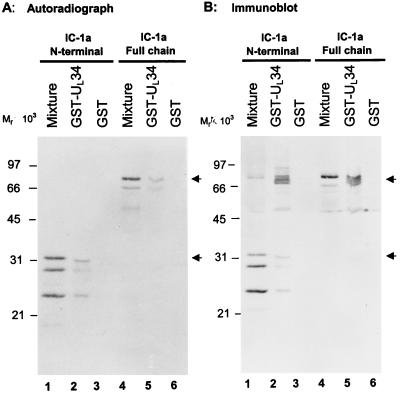
FIG. 5.
Reciprocal affinity precipitation of dynein IC-1a by GST-UL34 fusion protein. (A) Autoradiographic images of electrophoretically separated, [35S]methionine-labeled proteins from the in vitro translation reaction mixture of IC-1a bound to GST or GST-UL34 fusion proteins. In vitro-translated IC-1a whole chain or its N-terminal domain was reacted with GST or GST-UL34 fusion protein bound to glutathione-agarose beads. After being rinsed with binding buffer, the proteins bound to the beads were solubilized, subjected to electrophoresis on an SDS–12% polyacrylamide gel, transferred to a nitrocellulose sheet, and exposed to X-ray film. (B) Photograph of an immunoblot of proteins in vitro translated from IC-1a and bound to GST or GST-UL34 fusion proteins. The same blot from panel A, after being exposed to X-ray film, was reacted with polyclonal antibodies to IC-1a. Lanes 1 and 4, in vitro transcription-translation reaction mixtures of the N-terminal domain and the whole chain of IC-1a, respectively; lanes 2 and 5, in vitro-translated proteins bound to GST-UL34; lanes 3 and 6, in vitro-translated proteins bound to GST.