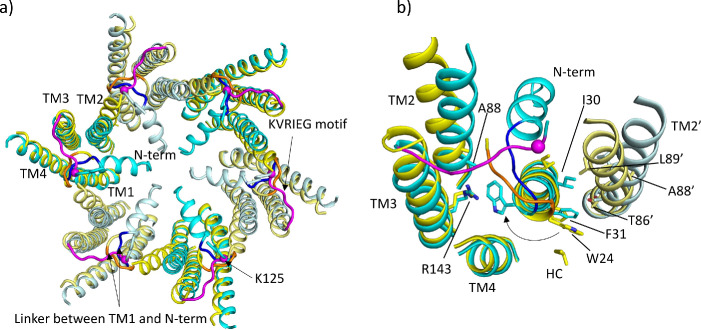
Figure 2. Comparison of lauryl maltose neopentyl glycol (LMNG)-NConst and LMNG-NFlex structures.
(a) Overall superposition showing the movement of TM2 and the link between the N-terminus and TM1. LMNG-NConst in cyan and LMNG-NFlex in yellow (alternate subunits have been coloured in lighter shades). The KVRIEG motif has been coloured magenta with a sphere indicating the position of K125. The residues between the N-terminus and TM1 for the LMNG-NConst structure have been coloured blue. (b) As (a) but focussed on TM1. The conformation of TM1 differs between the two structures as seen by the change in position of Trp24. TM2’ is from the neighbouring subunit. HC denotes the hydrocarbon chain from a lipid. The positions of T86’ and L89’ of TM2 in the NFlex conformation are not compatible with F31 and I30 TM1 in the NConst conformation.