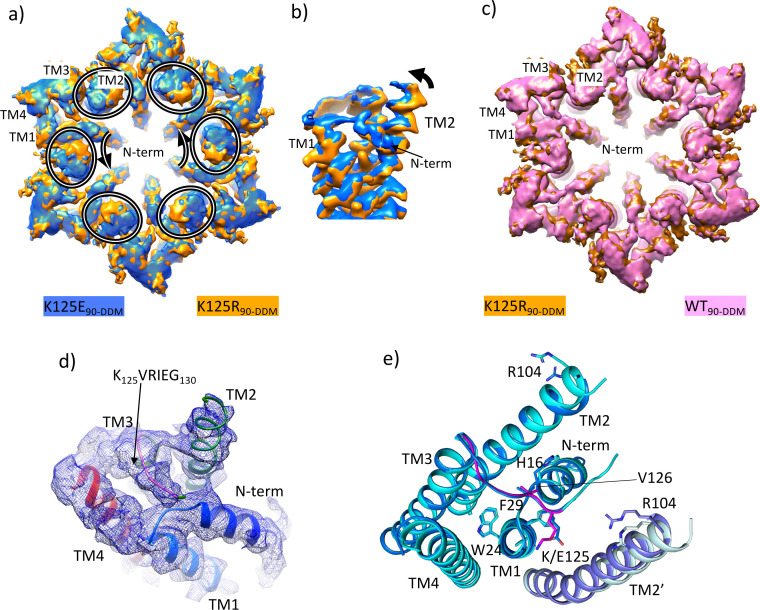
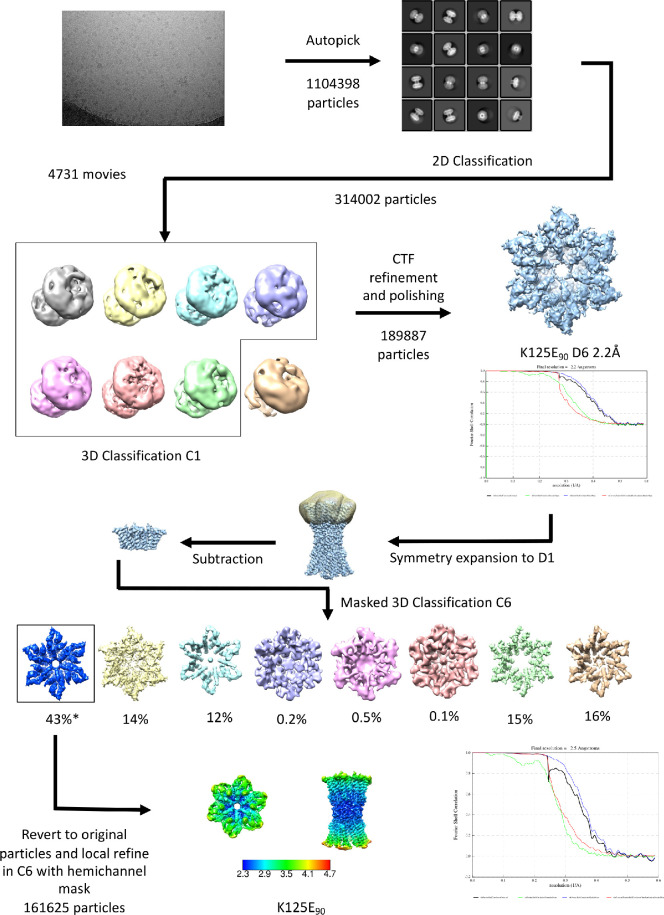
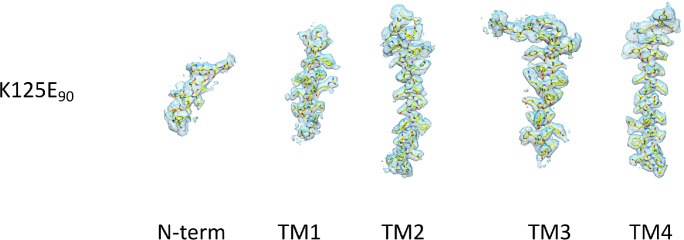
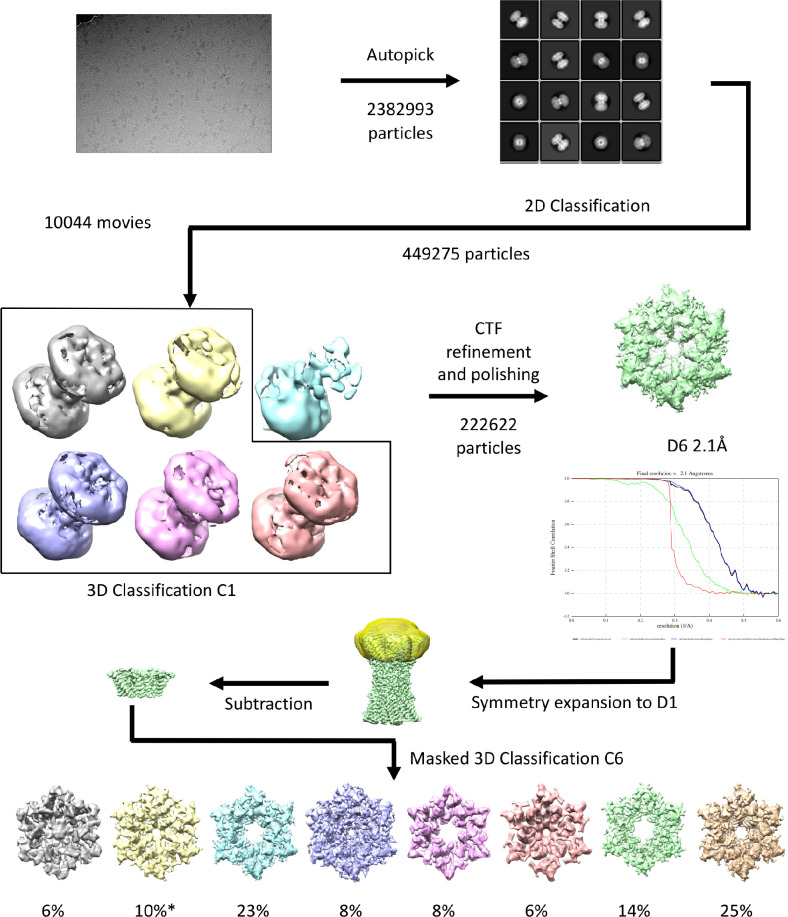
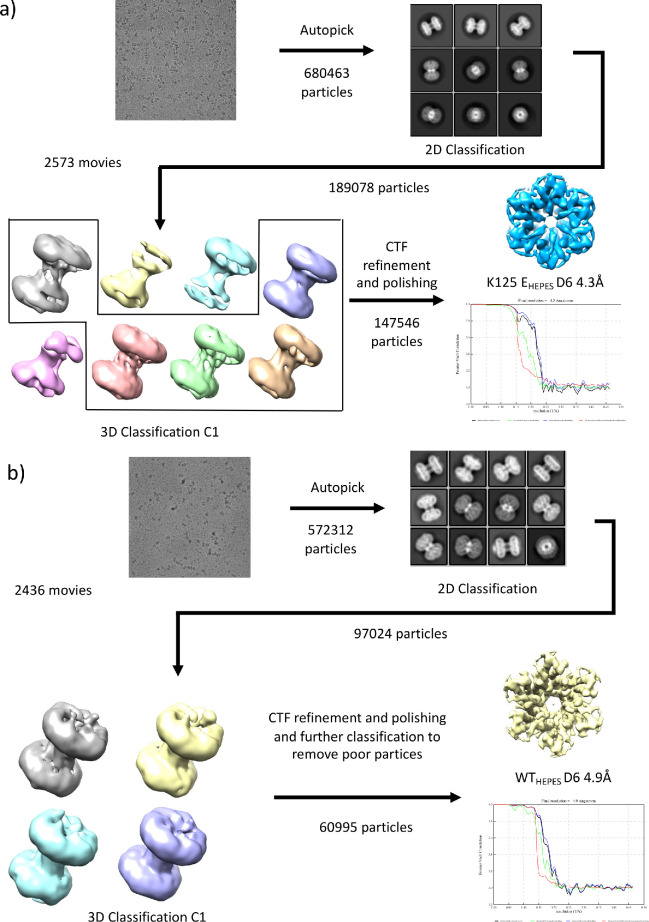
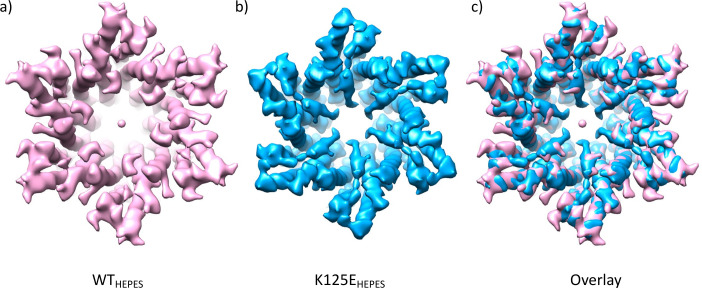
Figure 5. Density associated with the K125E mutant.
(a) Superposition of density for K125E90 D6 averaged map (blue) on the density for the K125R90 D6 averaged maps (orange). The ovals show the position of TM2 from each subunit and the arrows show the direction of the difference between TM2 in the two structures. (b) As (a) but focussed on TM2 in a view approximately perpendicular to the membrane. (c) Superposition of density for WT90 connexin26 (Cx26) (PDB ID 7QEQ; pink) D6 averaged maps on the density for R125E90 (orange). (d) Density associated with one subunit of the K125E90 structure (unsharpened map). The structure has been coloured as in Figure 1c. (e) Superposition of K125E90 structure (light blue) on the structure of lauryl maltose neopentyl glycol (LMNG)-NConst (cyan) showing the similarity between the two structures.