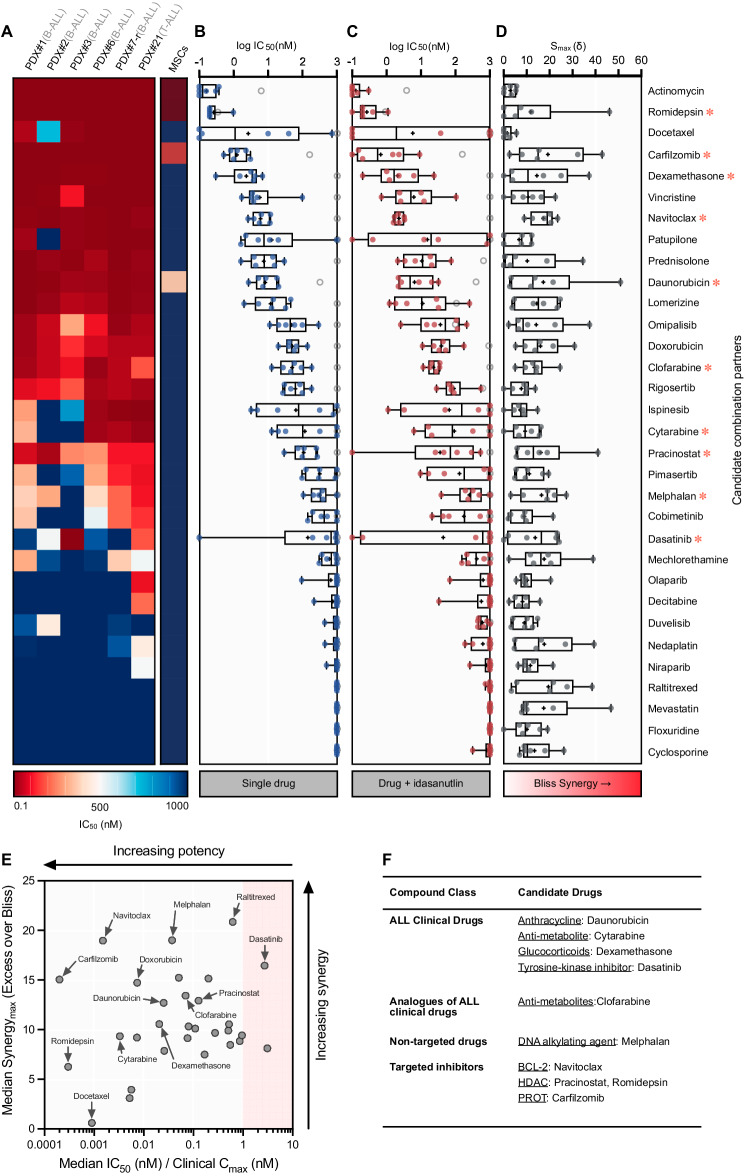
Fig. 2. Combinatory high-throughput drug repurposing library screening with 1971 FDA-approved drug compounds identifies potential synergistic idasanutlin combination partners.
A Heat map depicting drug concentrations resulting in 50% cell death (IC50) of 32 combination candidates determined using a 5-point log10-fold dilution range in a panel of 6 PDX leukemic samples. Each column represents an individual leukemia PDX sample, and each row represents a unique drug. Mean cell viability was determined in technical triplicate after 96 h drug exposure using fluorescence image-based microscopy (N = 1). Absolute live cell counts were normalized to respective vehicle (DMSO) controls. Drugs chosen for further investigation are indicated by a red asterisk ( ). B Box plot showing the distribution of mean IC50 values across each sample (N = 1). Mean MSC IC50 is indicated as a grey ring (N > 3) or bound to the highest concentration if > 1 μM. Boxes represent 25th to 75th percentiles, with median as a solid line, mean as a ‘+’, and whiskers extending to the range. C Box plot showing the mean IC50 for each PDX sample exposed to each library drug in combination with their respective idasanutlin doses resulting in approximately 40% cell death (IC40). Data were normalized to idasanutlin-treated cells to specifically examine the additive effects of the library drugs. D Distribution of maximum attained excess over Bliss synergy scores (Smax) for each drug in combination with respective idasanutlin IC40 and IC60 concentrations. E Dot plot showing the median IC50 of all PDX as a fraction of the clinically-reported Cmax for the respective drugs versus the median Smax score of all PDX samples. Clinically-reported pharmacokinetics and sources are provided in Supplementary Table S7. The red-shaded region indicates drugs with a median IC50 greater than clinically-reported limits. Results for mechlorethamine and mevastatin were omitted as clinical pharmacokinetic properties are unavailable. F Most promising drug combination candidates selected for further investigation.
). B Box plot showing the distribution of mean IC50 values across each sample (N = 1). Mean MSC IC50 is indicated as a grey ring (N > 3) or bound to the highest concentration if > 1 μM. Boxes represent 25th to 75th percentiles, with median as a solid line, mean as a ‘+’, and whiskers extending to the range. C Box plot showing the mean IC50 for each PDX sample exposed to each library drug in combination with their respective idasanutlin doses resulting in approximately 40% cell death (IC40). Data were normalized to idasanutlin-treated cells to specifically examine the additive effects of the library drugs. D Distribution of maximum attained excess over Bliss synergy scores (Smax) for each drug in combination with respective idasanutlin IC40 and IC60 concentrations. E Dot plot showing the median IC50 of all PDX as a fraction of the clinically-reported Cmax for the respective drugs versus the median Smax score of all PDX samples. Clinically-reported pharmacokinetics and sources are provided in Supplementary Table S7. The red-shaded region indicates drugs with a median IC50 greater than clinically-reported limits. Results for mechlorethamine and mevastatin were omitted as clinical pharmacokinetic properties are unavailable. F Most promising drug combination candidates selected for further investigation.