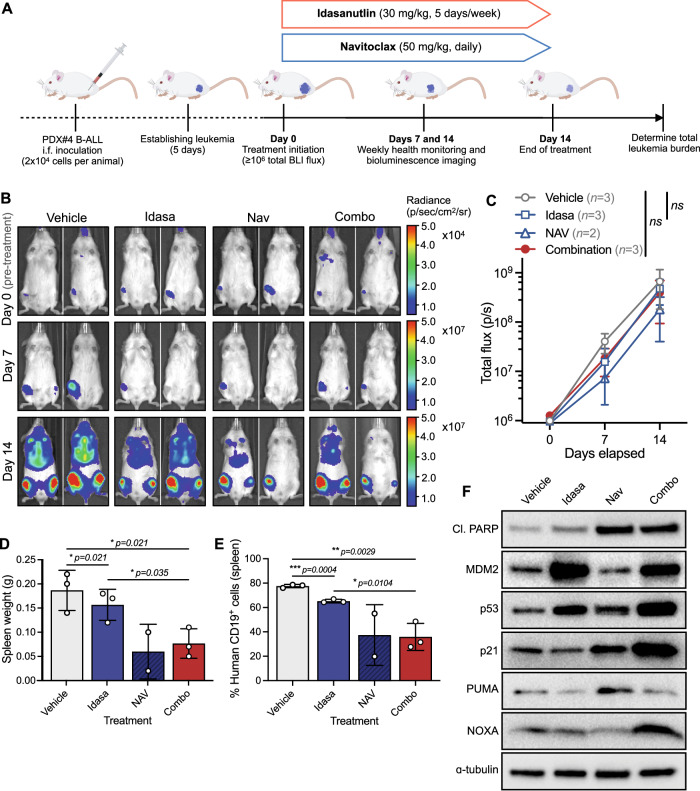
Fig. 7. Combination of idasanutlin and navitoclax inhibits in vivo growth of a very high-risk TCF3::HLF-rearranged ALL xenograft model.
A Schematic of experimental approach. B NSG mouse recipients received 2 × 104 viable B-ALL PDX#4 cells lentivirally transduced with pUltra-Chili-Luc plasmid to express Firefly luciferase. Engraftment was confirmed by bioluminescence imaging (BLI) 5 days after intrafemoral leukemia cell inoculation. Representative BLI results for days 0 (prior to any treatment), 7, and 14 after start of treatment of recipients treated with idasanutlin (30 mg/kg) once daily five days per week, navitoclax (50 mg/kg) once daily, and their combination for 14 days by oral gavage (p.o.). C Quantification of BLI by total flux in each treatment group. D Spleen weights from recipients four days after treatment with idasanutlin (30 mg/kg) once daily five days per week, navitoclax (50 mg/kg) once daily, and their combination for 14 days by oral gavage (p.o.). E Quantification of human CD19 + B-ALL cells by flow cytometry analysis of splenocytes from engrafted mice. F Steady-state pharmacodynamic analyses of spleen blasts derived from PDX#4 engrafted mice treated with each treatment arm for three consecutive days (n = 3 per group). Immunoblots show induction of cleaved PARP (Asp214), on-target p53 pathway signaling, and enhanced NOXA. ɑ-tubulin served as the loading control. In (C–E), treatment groups were compared by two-way ANOVA with Tukey post-hoc test and error bars indicate mean±s.d.