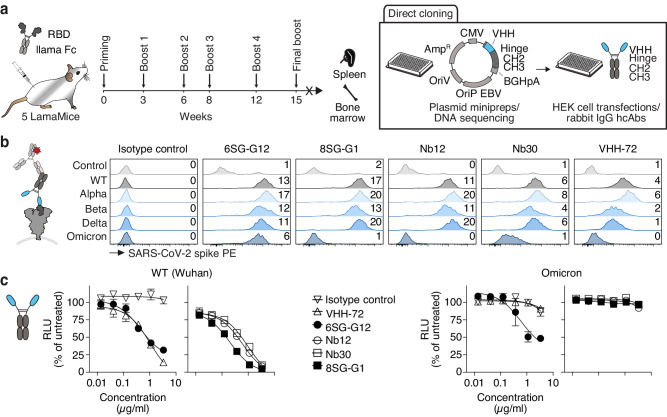
Fig. 4. Discovery of RBD-specific nanobodies from immunized LamaMice using direct cloning technology.
a LamaMice were immunized with a fusion protein comprising the recombinant receptor-binding domain (RBD) of the SARS-CoV-2 spike protein fused to the hinge, CH2 and CH3 domains of llama IgG2b. Three days after the final boost, the VHH-repertoire was PCR-amplified from cDNA prepared from spleen and bone marrow cells. PCR-amplicons were cloned into the pCSE2.5 expression vector upstream of the hinge, CH2 and CH3 domains of rabbit IgG. Plasmid DNA was prepared from individual colonies grown in 96-well plates, sequenced, and transiently transfected into HEK-6E cells cultivated in serum-free medium in 96-well plates. Supernatants were harvested 5 days after transfection. A liquots of the supernatants were analyzed for the production of heavy chain antibodies (hcAbs) by SDS-PAGE (see Supplementary Fig. 6a). b VHH-rabbit IgG hcAbs in HEK-6E supernatants were screened for binding to HEK293T cells transiently co-transfected with expression vectors for GFP and the spike protein of various SARS-CoV-2-strains. Bound antibodies were detected with PE-conjugated anti-rabbit IgG. Numbers indicate the mean PE fluorescence intensities (MFI x 10-3) of the GFP+ cell population. Parallel stainings were performed with VHH-rabbit IgG hcAbs containing nanobodies discovered by other groups from nanomice immunized with recombinant RBD (Nb12, Nb30) and a llama immunized with the spike protein of SARS-CoV-1 (VHH-72). c HEK293T cells stably overexpressing human ACE2 were incubated with luciferase-encoding lentiviral gene ontology vectors pseudotyped with SARS-CoV-2 spike protein of the wild type (WT) (Wuhan) or Omicron BA.2 variant in the presence of titrated amounts of the indicated VHH-rabbit IgG hcAbs. Two days after transduction, luciferase activity was quantified on a luminometer, 20 min after addition of luciferin. Data represent mean ± SD for triplicates.