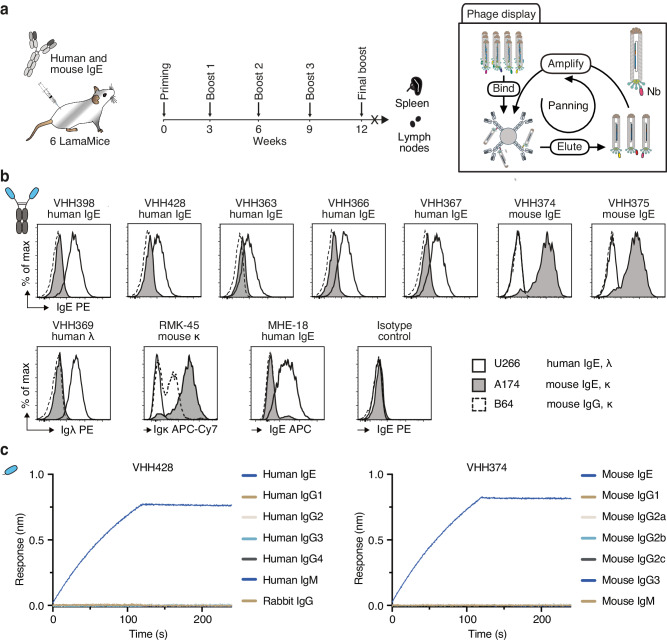
Fig. 5. Discovery of IgE-specific nanobodies from immunized LamaMice using phage display technology.
a LamaMice were immunized with a cocktail of human IgE and mouse IgE. Three days after the final boost, the VHH-repertoire was PCR-amplified from cDNA prepared from spleen and lymph nodes. PCR-amplicons were cloned into the pHEN2 phagemid vector upstream of the coding sequences for a His6x-c-Myc tag, an amber stop codon followed by the region encoding the gp3 surface protein of the M13 phage. Phage display libraries prepared from transformed E. coli were panned in solution on biotinylated IgE and complexes were captured on streptavidin beads. b The VHH-encoding region of enriched phages was sequenced and subcloned into the pCSE2.5 expression vector upstream of the hinge, CH2 and CH3 domains of rabbit IgG as in Fig. 4a. Specific binding of VHH-rabbit IgG hcAbs to cell lines expressing B cell receptors composed of human IgE (myeloma U-266), mouse IgE (hybridoma M1-3A174), or mouse IgG2a (M261B64) was analyzed by flow cytometry. Bound antibodies were detected with PE-conjugated anti-rabbit IgG. Control stainings were performed with anti-human Igλ (VHH369-rabbit IgG hcAb) or a VHH-rabbit IgG hcAb isotype control followed by PE-conjugated anti-rabbit IgG, and with directly conjugated anti-mouse Igκ (RMK-45) or anti-human IgE (MHE-18). c Nanobodies VHH428 and VHH374 were biotinylated and captured on streptavidin-coated biosensors. Specific binding to IgE and the other indicated Ig isotypes was analyzed by biolayer interferometry.