Abstract
Type 2 diabetes (T2D) is implicated as a risk factor for Alzheimer’s disease (AD), the most common form of dementia. In this work, we investigated neuroinflammatory responses of primary neurons to potentially circulating, blood–brain barrier (BBB) permeable metabolites associated with AD, T2D, or both. We identified nine metabolites associated with protective or detrimental properties of AD and T2D in literature (lauric acid, asparagine, fructose, arachidonic acid, aminoadipic acid, sorbitol, retinol, tryptophan, niacinamide) and stimulated primary mouse neuron cultures with each metabolite before quantifying cytokine secretion via Luminex. We employed unsupervised clustering, inferential statistics, and partial least squares discriminant analysis to identify relationships between cytokine concentration and disease-associations of metabolites. We identified MCP-1, a cytokine associated with monocyte recruitment, as differentially abundant between neurons stimulated by metabolites associated with protective and detrimental properties of AD and T2D. We also identified IL-9, a cytokine that promotes mast cell growth, to be differentially associated with T2D. Indeed, cytokines, such as MCP-1 and IL-9, released from neurons in response to BBB-permeable metabolites associated with T2D may contribute to AD development by downstream effects of neuroinflammation.
Subject terms: Neuroscience, Systems biology
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the progressive loss of memory and cognitive impairment, which affects more than 6.7 million people in the United States1. There is not a single cause for AD, but rather a multitude of risk factors that are likely responsible, including age, genetics, family history, and pre-existing health conditions2–4. Substantial evidence suggests that people with type 2 diabetes (T2D), a chronic metabolic disease that affects the body’s ability to regulate and process glucose, have an increased risk for AD5,6. While dependent on environmental location and other lifestyle factors, up to 81% of people who have AD have T2D or impaired glucose levels5,7. Accounting for age, a shared risk factor in both diseases, T2D is a significant risk factor for the development of AD8. The incurred risk of AD from altered glucose metabolism has been demonstrated in both rodent and human studies9–11. Some studies pointed to the link between disease co-morbidity to impairment of insulin receptors, which is associated with decreased brain glucose metabolism12,13. Other reports demonstrated that chronic inflammation from diabetes results in altered levels of proinflammatory cytokines in the brain, which may serve as a risk factor for AD pathology14–16. Despite the clear association between the risk of AD progression with a history of T2D, the biological mechanisms in which T2D pathobiology promotes AD development are not well understood.
The blood–brain barrier (BBB) is a structure of tightly connected brain endothelial cells, pericytes, and astrocytes that protects the brain from harmful substances and ensures the passage of nutrients from circulation into the brain17,18. The BBB also regulates the entry of immune cells into the central nervous system and the export of toxic metabolic waste from the brain19–21. When the BBB is impaired, substances that may not typically be transported to the brain, such as circulating metabolites, are more likely to cross over and stimulate local neuronal cells22. When stimulated by external factors such as metabolites, neurons and glia cells of the central nervous system may release cytokines to signal proinflammatory or anti-inflammatory responses23,24.
The breakdown of the BBB, as well as the disruption of metabolic regulation is observed in both AD and T2D18,25,26. In cases of AD, metabolic pathways such as the oxidative phosphorylation, glycolysis, and lipid metabolism are found to contribute to BBB dysregulation27,28. Additionally, signaling systems such as the nuclear factor-kappa B (NF-kB) and mammalian target of rapamycin are reported to be influence BBB integrity in AD29. Similar to AD, T2D is also acknowledged as a contributing factor to BBB disruption30. Studies report that disturbances to biological networks such as hexosamine, polyol pathways, and protein kinase C are associated with BBB integrity31,32. The protein kinase C pathway is shown to alter signaling of the mitogen activated protein kinase (MAPK) and NF-kB pathways, which are important for the transcription of cytokines and other pro- and anti-inflammatory molecules33,34. These findings may suggest that chronic inflammation not only contributes to BBB breakdown, but serves an important pathway that links T2D and AD35,36.
The aim of our study was to examine cytokine responses to AD and T2D-associated metabolites and to better understand the overlapping pathophysiology in which T2D exacerbates AD development. In the case of T2D-associated AD development, metabolites or other small molecules originating elsewhere in the body and circulating in the blood may cross the BBB to stimulate the cells in the brain. This chronic, low-grade stimulation of neuronal cells may lead to downstream neuroinflammation, promoting the development of AD37–41. However, the potential of systemic circulating species already upregulated in T2D to promote neuroinflammation in the brain has been understudied.
In this work, we identified patterns of cytokine production in primary mouse neurons following stimulation by metabolites differentially produced in T2D and AD. We find that primary neurons differentially secrete cytokines in response to different metabolites based on associations to AD, T2D, or both. Collectively, our findings indicate that disease-associated metabolites and their interactions with neurons may serve an important role in the neuroinflammatory pathway and the potential biological pathway in which T2D increases the risk of AD development.
Results
Nine candidate metabolites were selected for primary neuron stimulation
We identified nine candidate metabolites for follow-up studies, which include lauric acid, asparagine, fructose, arachidonic acid, aminoadipic acid, D-sorbitol, retinol, L-tryptophan, and niacinamide for the stimulation of primary culture of neurons (Table 1).
Table 1.
Metabolites selected from literature for neuron stimulation.
| Metabolite | Association | Relation to disease | Ref |
|---|---|---|---|
| Lauric Acid | T2D↑ | Upregulated in people with T2D or pre-diabetes; Hepatic insulin resistance in mice | 42,43 |
| Asparagine | T2D↑ | Upregulated in people with T2D; Correlated to higher freezing behavior in mice | 44,45 |
| Fructose-6-Phosphate | AD↑, T2D↑ | High fructose diet led to spatial memory impairment; T2D-like phenotype (rats) | 46,47 |
| Arachidonic Acid | AD↑, T2D↑ | Increased arachidonic acid resulted in elevated Aβ; Risk of metabolic syndrome | 48,49 |
| Aminoadipic Acid | AD↑, T2D↑ | Positively correlated with freezing behavior (mouse); fourfold risk for T2D (human) | 45,50 |
| D-Sorbitol | AD↑, T2D↑ | Positively correlated with freezing behavior; Induced glucose intolerance in mice | 45,51 |
| Retinol (Vitamin A) | AD↓, T2D↓ | Downregulated in people with T2D; Downregulated in people with AD | 42,52 |
| L-Tryptophan | AD↓ | Depletion of L-tryptophan induces cognitive deficit; Reduced Aβ levels when increased | 53,54 |
| Niacinamide | AD↓ | Niacinamide prevented cognitive deficits in mice; Lower niacinamide increases AD risk | 55,56 |
Further details supporting the selected candidates such as the study design can be found in Supplementary Table S1. Supporting evidence of the metabolites having the ability to enter the brain through the BBB is referred in Supplementary Table S2.
Retinol and arachidonic acid trigger distinct cytokine response compared to other metabolites
Of the 23 cytokines quantified, eight had more than 25% of the measurements below the lower limit of quantification (G-CSF, IL-17A, IL-13, IL-2, IL-3, IL-4, IL-5, and TNF-α), and were excluded from the analysis. The data for the fifteen remaining cytokines were pre-processed by replacing quantified values that were below the lowest limit of quantification with the lowest respective standard value (2.1% of data).
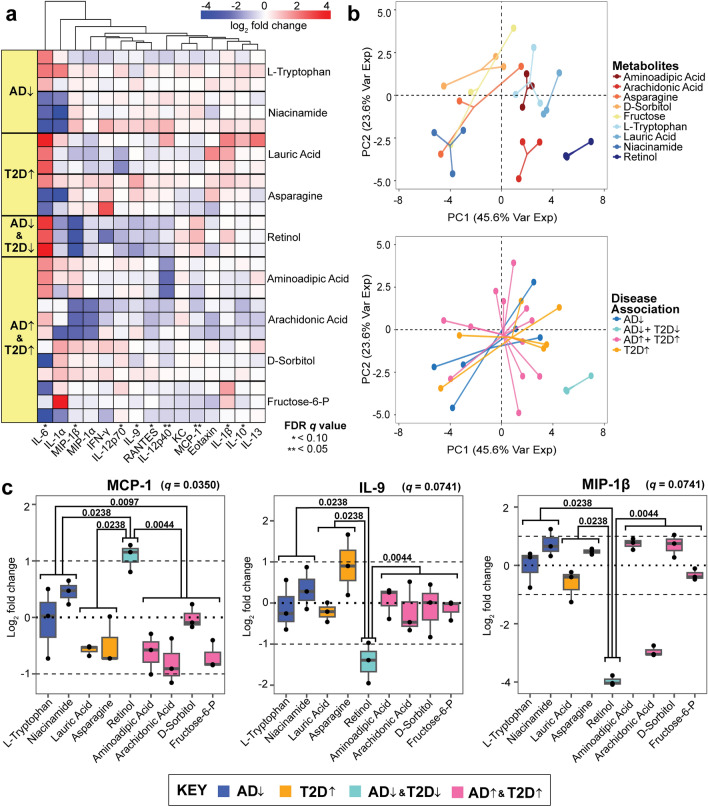
Here, we found that MCP-1, a chemoattractant for monocytes that enhances the recruitment of peripheral immune cells, was upregulated by retinol, L-tryptophan, and niacinamide, all of which are AD-protective metabolites57. All metabolites associated with T2D or AD downregulated MCP-1, whereas the metabolites associated with protective characteristics of T2D and AD upregulated MCP-1 (Fig. 1a). We found that retinol, which is associated with AD/T2D-protective properties, tended to induce larger magnitude fold changes in the cytokines compared to other metabolites. Nine cytokines showed statistically significant differences in abundance between the disease-association groupings of metabolites (Kruskal–Wallis test corrected by Benjamini–Hochberg FDR q value < 0.10), including IL-6, MIP-1β, IL-12p70, IL-9, RANTES, IL-12p40, MCP-1, IL-1β, and IL-10 (Fig. 1, Supplementary Fig. S1).
Figure 1.
Unsupervised hierarchical clustering of cytokines released by primary neurons (a) Hierarchical clustering of the log2 fold change of cytokine concentrations across the nine tested metabolites. Each metabolite is associated with a category of disease, with respective cells representing a quantified replicate. Significance was determined from a Kruskal–Wallis test corrected by the Benjamini–Hochberg method (FDR q value < 0.10). The direction of the arrows indicates disease (↑) and protective (↓) characteristics related to AD and T2D. (b) Principal component analysis of the log2 fold changes categorized by individual metabolites and disease-associations. (c) The log2 ratio of cytokine concentration to vehicles of MCP-1, IL-9, and MIP-1β. Mann–Whitney pair-wise testing was applied to each metabolite group based on disease association (p value denoted within the plot, with significance defined as p value < 0.05). The FDR q value from the corrected Kruskal–Wallis is displayed next to each respective cytokine.
We performed a principal component analysis (PCA) to determine which metabolites induced a different neuroinflammatory signaling response on the primary mouse neurons. We found that seven of the nine metabolites, excluding retinol and arachidonic acid, elicited cytokine responses that clustered regardless of disease association (Fig. 1b).
Retinol in particular, induced significant downregulation of IL-9 and MIP-1β and up-regulation of MCP-1 in a pattern that deviated significantly (Mann–Whitney test, p value < 0.05) from all other cytokine responses to other metabolites (Fig. 1c). These three cytokines contain at least one significant response difference between protective and detrimental associations of metabolites that are not consistent with MCP-1. The up-regulation of MCP-1 by retinol in the AD↓T2D↓ (protective) group, but not the AD-protective group alone, indicates that the retinol-MCP-1 response is specific to the T2D-AD axis, and not AD alone.
Multivariate statistical modeling identifies MCP-1 and IL-9 as T2D differentiating cytokines for AD development
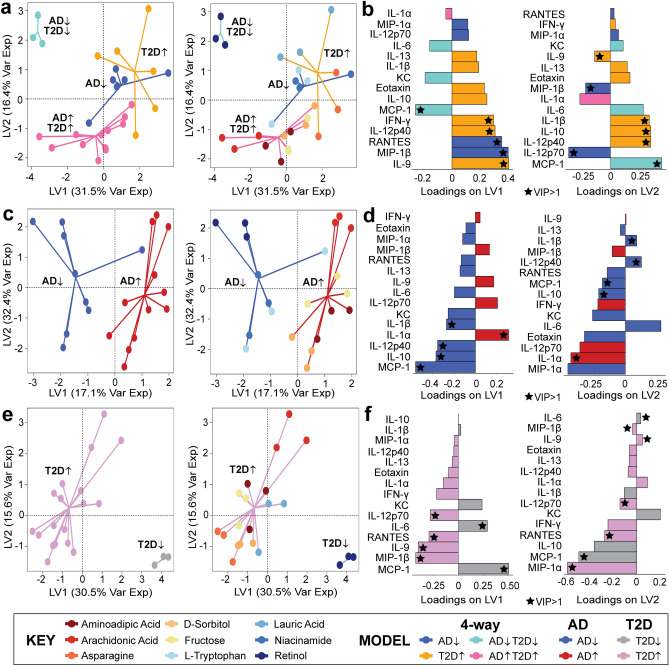
We next assessed whether a supervised modeling approach would identify combinations of cytokines capable of stratifying the metabolites based on different disease association groups. Using a multivariate statistical modeling framework: Partial Least Squares Discriminant Analysis (PLS-DA), we identified cytokines that are most predictive of either protective or disease properties of the disease association of interest. These metabolites were categorized with their respective disease associations: AD and T2D protective (AD↓T2D↓), AD and T2D (AD↑T2D↑), AD protective (AD↓), and T2D (T2D↑). The first model was to identify patterns of cytokine secretion that differentiated between AD-protective, AD/T2D-protective, T2D, and AD/T2D, answering the question if certain cytokines are more differentially produced in the presence of specific disease-associated metabolites. Here, we prepared two other models that separated the metabolites into AD- and T2D-only groups. For the latter two models, any metabolites that did not fit the criteria (e.g., T2D-only metabolite for the AD model) were excluded before applying the cross-validation and constructing the PLS-DA model.
In the 4-way PLS-DA model, we found that cytokines contributing to the overall PLS-DA model with a higher-than-average variable importance in projection (VIP, a higher-than-average defined as VIP > 1) on both LV1 and LV2 were MCP-1, IL-12p40, and IL-9. (Fig. 2a,b). A variable with a VIP > 1 in both LV1 and LV2 suggests that the specific cytokine is consistently contributing to the separation across different components of the PLS-DA model. We also found that the AD↓T2D↓ group separated from the other three other metabolite groups. This indicated that the T2D-AD protective metabolite, retinol, induced a distinct cytokine response while the other three groups, AD-protective, T2D-associated, and AD/T2D-associated, were more similar to each other.
Figure 2.
Separation of different disease-associated metabolites is detected from the PLS-DA model. (a,b) Four-way disease associations; (c,d) AD-protective and AD associated classifications, and (e,f) T2D-protective and T2D associated classifications. Loading variables for each model (LV1 and LV2) with a VIP > 1 is labeled with a star, and the color of the loading bar represents the cytokine with the highest contribution to the specific class (metabolite grouping).
Having demonstrated a separation among the four-way disease-association classification (Fig. 2a,b), we adjusted the model to compare metabolites based on their association to specific relationships, such as AD/AD-protective or T2D/T2D-protective. We adjusted our model to determine if our findings from the four-way model were consistent across the AD/AD-protective and T2D/T2D-protective models. In our AD-only model (Fig. 2c,d), the model separated between the AD disease-associated metabolites and the AD-protective metabolites across LV1. Cytokines with VIP > 1 contributing to both LV1 and LV2 were IL-1β, IL-12p40, IL-10, IL-1α, and MCP-1. Similar to the AD PLS-DA model, our T2D PLS-DA model successfully separated the metabolites associated with the protective and detrimental properties of T2D (Fig. 2e,f). In our T2D model, six cytokines had a VIP > 1 contributing to LV1 and LV2 (IL-12p70, RANTES, IL-6, IL-9, MIP-1β, and MCP-1).
Of the several cytokines contributing a VIP > 1 to each of the models, we identified two cytokines that were consistent across each of the three PLS-DA models. The two cytokines were MCP-1 and IL-9. MCP-1 was found to be contributing to the protective properties of T2D and AD, while IL-9 was identified to be contributing to T2D properties.
Discussion
Here, we used an in vitro primary neuron culture approach with the goal of examining shared and distinct cytokine responses to better understand the overlapping pathophysiology of T2D and AD. In particular, we studied the possibility of BBB-permeable metabolites with AD or T2D associations as a route for neuroinflammatory or protective processes in neurons associated with disease. Independent research groups have shown that dysregulation of the BBB is a known characteristic shared in both AD and T2D, with others studying the relationship between cytokine levels and disease status in separate AD and T2D studies25,26,58,59. Breakdown of the BBB may serve as an important connection between AD and T2D by allowing circulating factors to cross the BBB and induce disease-associated signaling cascades or provoke local inflammatory responses37–41.
Our work shows that MCP-1 is responsive to the AD/T2D protective metabolite retinol. Upregulation of MCP-1 corresponded to protective properties of AD and T2D, whereas downregulation of MCP-1 from primary neurons was a response to disease-associated metabolite stimulation. MCP-1 contributed more than average to each of the three PLS-DA models and MCP-1 was found to be responsive to metabolites associated with protective characteristics. Previous studies that investigated the relationship between serum retinol and AD reported diminished circulating retinol as an increased risk factor for cognitive decline in humans60 and in mice61. Additionally, retinol was found to be decreased in subjects with T2D, as well as subjects with T2D and diabetic retinopathy compared to healthy groups62. This case may be attributed to a derivative of retinol, retinoic acid, which is converted through a two-step oxidation process. Retinoic acid has been demonstrated to restore the insulin function of β-cells in mouse models63. β-cells are localized in the pancreas and are responsible for creating insulin to regulate blood sugar levels. Further understanding the possible protective role of retinol may serve as a viable preventative avenue for T2D-driven AD.
Through literature, we find evidence supporting that a deficiency of MCP-1-activated immune cells, such as microglia, have an increased risk of early onset of AD64. We hypothesize that in early-stage AD pathogenesis, neuron-secreted MCP-1 activates microglia to promote the clearance of amyloid-beta (Aβ) proteins in the brain, and based on our results, retinol stimulation could promote this process65–67. This finding is promising because present in people with AD, Aβ, and tau tangles are the hallmark proteins found aggregated in the brain68,69. However, prolonged activation of microglia may reduce phagocytic efficiency which may result in downstream damage to neurons through the accumulation of Aβ, eventually leading to AD development70,71.
Despite our findings, other sources that have studied AD signaling found MCP-1 to be associated with longitudinal cognitive decline in patients with AD72,73. However, MCP-1 from these studies quantified cytokines from plasma samples, rather than neuron-derived media. Additionally, these observations were made in later stages of AD, which may suggest that the change in MCP-1 regulation occurs in cases of mild to severe AD. Thus, we acknowledge that there may be conflicting cytokine findings from human AD studies72–75. This difference in results may be attributed to several factors, such as the pathophysiological stage of the disease, high dimensionality of human biology, and the location in the body to which the cytokines were quantified.
IL-9 was responsive to T2D↑ associated metabolites (lauric acid and asparagine) and significantly differentially abundant compared to retinol, the AD↓T2D↓ metabolite. IL-9 is responsible for a large variety of physiological processes, including the promotion of mast cell growth and function, similar to MCP-176. For both of these metabolites, PLS-DA also revealed IL-9 to be contributing more than average to the classification of T2D. In the context of differential cytokine responses to AD or T2D, IL-9 signaling may be more associated with T2D, potentially suggesting that IL-9 signaling may not be responsible for the shared development between AD and T2D. A study by Mohammed et al., found that serum IL-9 in T2D patients was higher than that of the healthy control group77. A separate study found that lower serum IL-9 was associated with pre-diabetes and T2D78. The conflicting results are based on cytokine quantification from serum samples and may not be reflective of the responses we observed in neuronal media. Though IL-9 was not significantly responsive to the specific AD-associated metabolites we tested, others found that higher neuron and astrocyte production of IL-9 in vitro is linked to AD progression79 and this is also observed in human studies80.
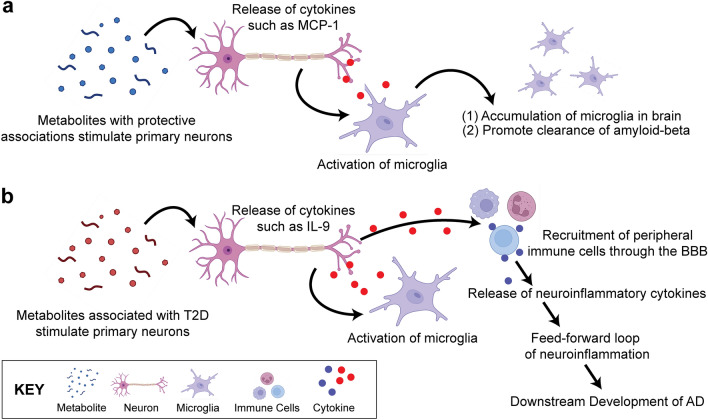
Based on our findings, we hypothesize a potentially shared neuroinflammatory response pathway where, during a healthy state (Fig. 3a), the BBB regulates the passage of nutrients and substances across the membrane with a tightly locked layer of brain endothelial cells. As a result, upregulated metabolites due to metabolic disease development will have a difficult time crossing the BBB to stimulate the neuronal cells. This ultimately prevents peripheral immune cells from also entering the brain area which will generate a larger neuroinflammatory response. Some signaling networks that involve MCP-1 include the MAPK81 and NF-kB82 pathways. The c-Jun N-terminal kinases (JNK) pathway, a subtype of MAPK, is capable of mediating neuroinflammation and the eventual breakdown of the BBB83. Thus, regulated MCP-1 may play an important role in AD development. In cases of a diseased state, the BBB breaks down, and harmful substances and unwanted cells can leak across the membrane (Fig. 3b). As a result, neuronal cells become stimulated and release cytokines84. These cytokines can be pro-inflammatory or anti-inflammatory and trigger other downstream effects that may lead to a positive feedback loop of cytokine secretions. This long-term cycle may ultimately contribute to the disruption of the BBB, as well as chronic neuroinflammation we may observe in people living with AD today. Further investigation of these pathways may improve our understanding of T2D and AD.
Figure 3.
Hypothesized Pathway of the Shared Neuroinflammatory Response. (a) In a healthy state, few metabolites may cross the BBB through specialized transport. The stimulated neurons produce cytokines that may activate glia such as microglia, which will accumulate in the brain and promote clearance of amyloid-beta and other debris in the central nervous system. (b) In a diseased state, metabolites may cross the BBB in higher concentrations, and stimulate the neurons and glia cells. This chronic, low-grade stimulation of neuronal cells may result in the eventual breakdown of the BBB, leading to downstream migration of immune cells and more metabolites to enter the brain. The release of neuroinflammatory cytokines may generate a feed-forward loop of neuroinflammation, potentially leading to the development of eventual AD. (Created with BioRender.com).
A limitation of our work is that the neuron culture only represents one of the many cells located in the central nervous system that models a single-direction pathway where neurons respond to different disease-associated metabolites. While it is important to note that astrocytes and microglia are important sources of cytokine production, increasing evidence suggests that neurons can also produce inflammatory molecules85–87. Other studies have also investigated neuron cytokine production in both AD88 and T2D89. We also note that we did not include mitotic inhibitors in our cell culture due to concerns about alteration of cellular response due to partial toxicity. Lack of mitotic inhibitors can result in astrocyte contamination of neuronal cultures despite careful visual inspection90,91. It is thus possible that some of the measured cytokines are astrocytic in origin rather than neuronal. However, given our goal of identifying disease-relevant metabolites produced by the body with the capability of crossing the BBB and affecting the neuroimmune environment of the brain, we do not consider the presence of astrocytes in our culture to be detrimental to this goal. While we are primarily concerned with neuronal response to these metabolites as AD is a neurodegenerative disease, AD also profoundly alters glial cells, which indirectly affect neuron health through changes in their regime of neuronal support92,93. In the future, preparing an experiment with different co-culture models may pose alternative methods to study signaling networks related to T2D as a risk factor for AD development.
Additionally, there are limitations in our assumptions. Cytokines are complex signaling molecules that themselves cannot be categorically placed into simple disease associations. Likewise, it is difficult to fully classify a metabolite to be associated with a specific disease, as human biology is multi-dimensional. We also acknowledge that the category of upregulation and downregulation of these metabolites do not necessarily suggest that the metabolites themselves are protective or detrimental but may represent the endpoint of downstream intracellular process. This also includes the limitation of achieving physiologically relevant metabolite concentrations in the brain, especially with our assumption that AD can be approximated with acute administration at high concentration. While we estimate the concentration ranges based on literature (Supplementary Table S3) and confirm cell viability with a live-dead assay (Supplementary Fig. S2), there may be implications in translating direct concentrations to human conditions.
Our work examined the patterns of neuroinflammatory cytokines released by primary mouse neurons when stimulated by metabolites differentially produced in AD and T2D. Our findings show that metabolites with similar disease associations result in similar profiles of differentiating cytokines such as MCP-1. Understanding the patterns of cytokines released by neuronal cells will allow us to infer the potential neighboring cells that may be activated. Our results suggest a need for further studies to investigate T2D-driven neuroinflammation as a contributor to AD.
Materials and methods
Candidate metabolite selection
The selection criteria for inclusion of metabolites in our study were (1) that the metabolite be differentially abundant in AD, T2D, or both, (2) that the metabolite be BBB-permeable, and (3) that two or more studies supported these associations. We used the search terms “metabolites present in Alzheimer’s disease” and “metabolites present in type 2 diabetes” in Google Scholar and PubMed. From the search, longitudinal or metabolomic studies (both human and mouse) were first prioritized, with candidate metabolites identified from key results or available data from publications. The identified metabolites were then given an association with detrimental or protective characteristics based on findings from at least two studies. The associations were established by using search terms “[identified metabolite name] and Alzheimer’s disease” and “[identified metabolite name] and type 2 diabetes.” In our search criteria, while there is an increased BBB permeability shared in both T2D and AD, all metabolites were verified to have the ability to cross the blood–brain barrier based on specialized transport mechanisms or favorable chemical properties. We confirmed this through literature findings with the keyword search “[metabolite name] and BBB permeability”94–102. Metabolite associations were confirmed with at least two studies. Studies reporting contradictory results for identified metabolites were avoided for this study.
Animal use and ethics approval
This study is reported in accordance with ARRIVE guidelines. All animal procedures were performed in strict accordance with the guidelines approved by the Penn State College of Medicine Institutional Animal Care and Use Committee (IACUC) (PROTO201800449). The Penn State College of Medicine IACUC is the institutional committee responsible for all ethical approvals of research involving vertebrate animals and this study is approved under protocol PROTO201800449.
Primary neuron culture
The primary neurons used in this investigation are derived from embryonic litters from pregnant CD-1 mice (Charles River Laboratories, strain 022). Two pregnant mice (gestational day 17) were sacrificed by decapitation using a guillotine. Between the two pregnant mice, a total of 25 embryonic pups were sacrificed by decapitation using surgical scissors. The isolated brains were immediately placed in cold HEPES-buffered Hank’s Balanced Salt solution for dissection. After the meninges were removed, the cortical cap of the brain was isolated.
Primary neuron culture was performed according to validated methods88. The isolated cortices were transferred to a conical tube containing warm embryonic plating medium: Neurobasal Plus (Gibco), 10% fetal bovine serum (Gibco), 1 × GlutaMAX (Gibco), 1 × Penicillin–Streptomycin (10,000 U/mL, Gibco). Cortices were manually triturated using a pipette in the embryonic medium, and the overall cell concentration was determined by the Countess II automated cell counter (Invitrogen). The 6-well plates coated with 0.1 mg/mL Poly-D-Lysine were plated with the cell suspension at a density of 5,196,500 cells/well. After 24 h, the embryonic media was replaced with neuronal media: Neurobasal Plus (Gibco), 1 × B27 Plus supplement (Gibco), 1 × GlutaMAX (Gibco), 1 × Penicillin–Streptomycin (10,000 U/mL, Gibco). Half of the neuronal medium was replaced after four days. After inspecting the plates for confluency and lack of visible contaminants, the neuronal medium was aspirated, and neurons were stimulated with metabolites dissolved in media on day 8 with a final concentration of 300 uM. On day 11, cell media was collected and snap-frozen in liquid nitrogen for future cytokine quantification with the Luminex system.
Established metabolite stimulation concentration
Metabolite treatment concentration was determined by dose–response neuron viability (Invitrogen, catalog no. L3224) in the nano and micromolar range. We established the range we tested for our live/dead assay based on the ranges tested in these metabolites with different cell lines in prior studies103–111 (Supplementary Table S3). Like the primary neuron culturing methods, one pregnant CD1 mouse at gestational day 15 (n = 12 embryos) was sacrificed and plated, with the adjustment of 175,000 cells/well in a 96-well plate. On day 8, we stimulated neurons with twelve logarithmically increasing concentrations (1 nM, 3 nM, 10 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1 uM, 3 uM, 10 uM, 30 uM, 100 uM, and 300 uM) (Supplementary Fig. S2). Each metabolite was first dissolved in dimethyl sulfoxide, water, or phosphate-buffered saline before being mixed with neuronal media. The vehicle controls (no metabolite added) matched the solvent composition respective to each metabolite group. The dimethyl sulfoxide concentration did not exceed 0.3% to ensure cell viability. On day 11, we assayed neuron viability using live/dead staining (calcein acetoxymethyl and ethidium homodimer, catalog no. L3224, Invitrogen). Fluorescence was measured using the Spectramax i3x microplate reader (Molecular Devices). We found no significant decline in cell viability as the concentration increased, thus, the 300 uM concentration was selected as the established concentration for our assays, with the assumption and caveat that high acute exposure will exhibit a similar response to low chronic exposure.
Cytokine quantification
Samples, blanks, and quality controls were loaded into a 384-well plate in technical triplicate, and cytokine concentrations were quantified using the Luminex Bio-Plex 3D platform. The assay was carried out with the Bio-Rad BioPlex 23-Plex Pro Mouse Cytokine Kit, which contains a panel to quantify pro- and anti-inflammatory cytokines (Eotaxin, G-CSF, IFN-γ, IL-17A, IL-1β, IL-1α, IL-10, IL-12p40, IL-12p70, IL-13, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, KC, MCP-1, MIP-1β, MIP-1α, RANTES, and TNF-α) from Bio-Rad (Catalog no. M60009RDPD). The manufacturer’s protocol was modified to accommodate a 384-well format by adding magnetic beads and antibodies at a reduced volume112,113.
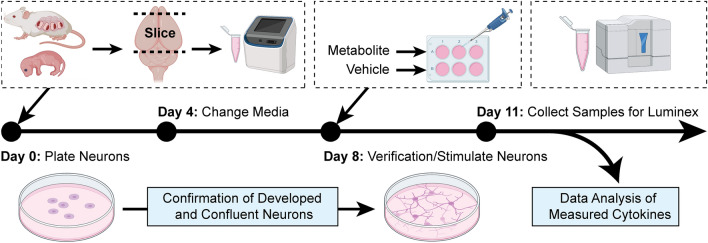
On the day of the assay, the collected samples were thawed on ice and standards were reconstituted and prepared via a four-fold dilution series. The magnetic bead solution, neuronal medium, standards, samples, and blanks were pipetted into the designated wells. The 384-well plate was then covered with a sealing covering tape and incubated on a shaker at 850 RPM in room temperature, for one hour. After washing, diluted detection antibodies were added into each well, followed by incubation and washing with wash buffer. Streptavidin–Phycoerythrin was multi-channel pipetted into each well after washing. All steps that involved plate washing were performed with the Hydrospeed plate washer with magnets (Tecan). The Luminex instrument calibration and verification were conducted on the day of the assay. The samples were then collected and quantified using the Luminex assay (Fig. 4).
Figure 4.
Method in plating and culturing primary neurons derived from embryonic CD1 mice. The embryonic litters from pregnant CD-1 mice were decapitated, and the cortical regions were isolated for primary neuron culturing. On day 8, the metabolites and respective vehicles were used to stimulate the primary neurons. After 3 days, the neuron media samples were collected for the quantification of released cytokines using the Luminex platform. (Created with BioRender.com).
Data pre-processing and normalization
Cytokines with 25% or more of the samples reading below the lower limit of quantification were excluded from the analysis. The remaining 15 quantified cytokines were retained for downstream analysis. To prepare the data for analysis, any individual values below the lower limit of quantification were replaced with the lowest respective standard value (2.1% of data). Cytokine concentrations were then normalized by a log2 ratio of the individual measurement divided by the average of the triplicate vehicle concentrations. All data analysis was conducted in RStudio (version 1.4.1717 Juliet Rose).
Unsupervised hierarchical clustering and principal component analysis
The log2 normalized data was used for unsupervised hierarchical clustering and principal component analysis (PCA). The PCA provides a dimensionally reduced visualization of data clustering between metabolite groups. For a global comparison across different metabolite groups and cytokines, a heatmap was generated using the pheatmap package in RStudio (package version 1.0.12). The factoextra package was used for PCA, with the input data scaled and normalized to the vehicle groups (package version 1.0.7).
Statistical analysis
To determine the statistical significance of differences in cytokine levels across metabolite treatment groups, we performed a non-parametric Kruskal–Wallis test on the data. A Benjamini–Hochberg was performed on the Kruskal–Wallis test to correct the false discovery rate (FDR) of multiple comparisons. Significance was determined if an FDR q value was less than 0.10. For each individual cytokine, we performed post-hoc testing with a non-parametric Mann–Whitney test to identify significantly differing responses across the four groups of disease-associated metabolites (AD-protective, AD/T2D-protective, T2D, and AD/T2D), with a p value less than 0.05 considered significant. Significantly differentially abundant cytokines were visualized with box and whisker plots.
Partial least squares discriminant analysis
We performed partial least squares discriminant analysis (PLS-DA), a multivariate dimensionality-reduction technique in RStudio using mixOmics (package version 6.16.3). For PLS-DA, the normalized log2 fold change of cytokine concentrations secreted by primary neurons were independent predictor variables and our dependent variable was the disease association of the metabolites. We constructed three PLS-DA models using cytokine profiles to predict (1) 4-way discrimination between all metabolite groups (AD-protective, AD/T2D-protective, T2D, and AD/T2D), (2) AD-protective vs. AD-associated, and (3) T2D-protective vs. T2D-associated metabolite stimulation conditions. The number of latent variables (LV) selected for each model was determined by a three-fold cross-validation repeated randomly one hundred times based on the model with the lowest cross-validation error rate. The purpose of this approach is to identify cytokines most predictive of different disease associations and their association to harmful or protective effects.
In our PLS-DA model, we identified the most important cytokines contributing to the model’s overall predictive accuracy by the VIP score. The VIP scores were calculated by using the mixOmics package, which represents the strength of contribution from each cytokine to the results of the PLS-DA model:
where K is the total number of cytokine predictors, A is the number of PLS-DA components, wak is the weight of predictor j in the ath LV component, and SSYa is the sum of squares of the explained variance for the ath LV component. The SSYtotal represents the total sum of squares explained in all of the LV components. Since the VIP accounts for normalization, a score greater than 1 indicates an important variable within the model.
Supplementary Information
Acknowledgements
This work is supported by an award from the Good Ventures Foundation and Open Philanthropy, as well as start-up funds from Purdue University Weldon School of Biomedical Engineering (DKB and BKB). This work is also supported by R21AG068532 from the National Institute on Aging (EAP). BKB is supported by the NIH T32 predoctoral fellowship T32DK101001 from the National Institute of Diabetes and Digestive and Kidney Diseases. BKB acknowledges the National Science Foundation for support under the Graduate Research Fellowship program (GRFP) under grant number DGE-1842166. MKK is supported by training fellowship T32NS115667 from the National Institute of Neurological Disorders and Stroke. RMF is supported by NIH NRSA predoctoral fellowship F31AG071131 from the National Institute on Aging. The authors thank Javier Muñoz Briones (Purdue University) for support in the Luminex assay. The authors also thank Raymond Krajci (Case Western Reserve University) for verifying reproducibility of the code used for analysis.
Abbreviations
- AD
Alzheimer’s disease
- BBB
Blood–brain barrier
- FDR
False discovery rate
- G-CSF
Granulocyte colony-stimulating factor
- IFN-γ
Interferon-γ
- IL
Interleukin
- KC
Keratinocyte chemoattractant
- LV
Latent variable
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MIP-1α
Macrophage inflammatory protein-1α
- MIP-1β
Macrophage inflammatory protein-1β
- NF-kB
Nuclear factor-kappa B
- PCA
Principal component analysis
- PLS-DA
Partial least squares discriminant analysis
- RANTES
Regulated on activation, normal T cell expressed and secretion
- T2D
Type 2 diabetes
- TNF-α
Tumor necrosis factor-α
- VIP
Variable importance in projection
Author contributions
BKB: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, visualization, writing-original draft, writing-review & editing. MKK: Formal analysis, investigation, methodology, writing-review & editing. RMFB: Formal analysis, investigation, methodology, writing-review & editing. EAP: Conceptualization, funding acquisition, methodology, project administration, resources, writing-review & editing. DKB: Conceptualization, funding acquisition, methodology, project administration, resources, writing-review & editing.
Data availability
Generated data for analysis is included and available in the Supplementary Files of this article.
Code availability
All code is publicly available at https://github.com/Brubaker-Lab/AD-T2D-Cytokine-Manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-62155-3.
References
- 1.Alzheimer’s Association 2023 Alzheimer’s disease facts and figures. Alzheimers Dementia. 2023;19:1598–1695. doi: 10.1002/alz.13016. [DOI] [PubMed] [Google Scholar]
- 2.Frigerio CS, et al. The major risk factors for Alzheimer’s disease: Age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 2019;27:1293–1306.e6. doi: 10.1016/j.celrep.2019.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschenbrenner AJ, Balota DA, Gordon BA, Ratcliff R, Morris JC. A diffusion model analysis of episodic recognition in preclinical individuals with a family history for Alzheimer’s disease: The adult children study. Neuropsychology. 2016;30:225–238. doi: 10.1037/neu0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y-WA, Zhou B, Wernig M, Südhof TC. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell. 2017;168:427–441.e21. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 23 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2015;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association 2017 Alzheimer’s disease facts and figures. Alzheimers Dementia. 2017;13:325–373. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 8.Barbiellini Amidei C, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA. 2021;325:1640–1649. doi: 10.1001/jama.2021.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okereke OI, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J. Am. Geriatr. Soc. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 10.Manschot SM, et al. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winocur G, et al. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 12.Bingham EM, et al. The role of insulin in human brain glucose metabolism. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 13.Hirvonen J, et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes. 2011;60:443–447. doi: 10.2337/db10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagarde J, Sarazin M, Bottlaender M. In vivo PET imaging of neuroinflammation in Alzheimer’s disease. J. Neural Transm. 2018;125:847–867. doi: 10.1007/s00702-017-1731-x. [DOI] [PubMed] [Google Scholar]
- 17.Blanchette M, Daneman R. Formation and maintenance of the BBB. Mech. Dev. 2015;138:8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Cai Z, et al. Role of blood-brain barrier in Alzheimer’s disease. J. Alzheimers Dis. 2018;63:1223–1234. doi: 10.3233/JAD-180098. [DOI] [PubMed] [Google Scholar]
- 19.Zenaro E, et al. Neutrophils promote Alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 20.Naert G, Rivest S. A deficiency in CCR2+ monocytes: The hidden side of Alzheimer’s disease. J. Mol. Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- 21.Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015 doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 25.Montagne A, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starr JM, et al. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Zhang J. Lipid metabolism in Alzheimer’s disease. Neurosci. Bull. 2014;30:331–345. doi: 10.1007/s12264-013-1410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das TK, Ganesh BP, Fatima-Shad K. Common signaling pathways involved in Alzheimer’s disease and stroke: Two faces of the same coin. J. Alzheimers Dis. Rep. 2023;7:381–398. doi: 10.3233/ADR-220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brook E, et al. Blood-brain barrier disturbances in diabetes-associated dementia: Therapeutic potential for cannabinoids. Pharmacol. Res. 2019;141:291–297. doi: 10.1016/j.phrs.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 32.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: An overview. J. Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Santani D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009;61:595–603. doi: 10.1016/S1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- 35.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/S0166-2236(00)02004-X. [DOI] [PubMed] [Google Scholar]
- 36.Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front. Neurosci. 2018 doi: 10.3389/fnins.2018.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 38.Heppner FL, Ransohoff RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 39.Hemonnot A-L, Hua J, Ulmann L, Hirbec H. Microglia in Alzheimer disease: Well-known targets and new opportunities. Front. Aging Neurosci. 2019;11:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017;127:3240–3249. doi: 10.1172/JCI90606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W, et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature. 2019;569:663–671. doi: 10.1038/s41586-019-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamoshita K, et al. Lauric acid impairs insulin-induced Akt phosphorylation by upregulating SELENOP expression via HNF4α induction. Am. J. Physiol.-Endocrinol. Metab. 2022;322:E556–E568. doi: 10.1152/ajpendo.00163.2021. [DOI] [PubMed] [Google Scholar]
- 44.Luo H-H, Feng X-F, Yang X-L, Hou R-Q, Fang Z-Z. Interactive effects of asparagine and aspartate homeostasis with sex and age for the risk of type 2 diabetes risk. Biol. Sex Differ. 2020;11:58. doi: 10.1186/s13293-020-00328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunsberger HC, et al. Divergence in the metabolome between natural aging and Alzheimer’s disease. Sci. Rep. 2020;10:12171. doi: 10.1038/s41598-020-68739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Memory. 2009;92:410–416. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balakumar M, et al. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol. Cell. Biochem. 2016;423:93–104. doi: 10.1007/s11010-016-2828-5. [DOI] [PubMed] [Google Scholar]
- 48.Amtul Z, Uhrig M, Wang L, Rozmahel RF, Beyreuther K. Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer’s disease: Structural insight. Neurobiol. Aging. 2012;33(831):e21–831.e31. doi: 10.1016/j.neurobiolaging.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Williams ES, Baylin A, Campos H. Adipose tissue arachidonic acid and the metabolic syndrome in Costa Rican adults. Clin. Nutr. 2007;26:474–482. doi: 10.1016/j.clnu.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang TJ, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013;123:4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C-H, et al. Long-term consumption of the sugar substitute sorbitol alters gut microbiome and induces glucose intolerance in mice. Life Sci. 2022;305:120770. doi: 10.1016/j.lfs.2022.120770. [DOI] [PubMed] [Google Scholar]
- 52.Dorey CK, Gierhart D, Fitch KA, Crandell I, Craft NE. Low xanthophylls, retinol, lycopene, and tocopherols in grey and white matter of brains with Alzheimer’s disease. J. Alzheimer’s Dis. 2023;94:1–17. doi: 10.3233/JAD-220460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter RJ, et al. Cognitive deficit induced by acute tryptophan depletion in patients with Alzheimer’s disease. AJP. 2000;157:638–640. doi: 10.1176/appi.ajp.157.4.638. [DOI] [PubMed] [Google Scholar]
- 54.Noristani HN, Verkhratsky A, Rodríguez JJ. High tryptophan diet reduces CA1 intraneuronal β-amyloid in the triple transgenic mouse model of Alzheimer’s disease. Aging Cell. 2012;11:810–822. doi: 10.1111/j.1474-9726.2012.00845.x. [DOI] [PubMed] [Google Scholar]
- 55.Green KN, et al. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalmasso MC, et al. Nicotinamide as potential biomarker for Alzheimer’s disease: A translational study based on metabolomics. Front. Mol. Biosci. 2023;9:1067296. doi: 10.3389/fmolb.2022.1067296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021;101:107598. doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taipa R, et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 59.Spranger J, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 60.Huang X, et al. Diminished circulating retinol and elevated α-TOH/retinol ratio predict an increased risk of cognitive decline in aging Chinese adults, especially in subjects with ApoE2 or ApoE4 genotype. Aging (Albany NY) 2018;10:4066–4083. doi: 10.18632/aging.101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Touyarot K, et al. A mid-life vitamin A supplementation prevents age-related spatial memory deficits and hippocampal neurogenesis alterations through CRABP-I. PLoS One. 2013;8:e72101. doi: 10.1371/journal.pone.0072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, et al. Relationship between retinol and risk of diabetic retinopathy: A case-control study. Asia Pac. J. Clin. Nutr. 2019;28:607–613. doi: 10.6133/apjcn.201909_28(3).0021. [DOI] [PubMed] [Google Scholar]
- 63.Brun P-J, et al. Retinoic acid receptor signaling is required to maintain glucose-stimulated insulin secretion and β-cell mass. FASEB J. 2015;29:671–683. doi: 10.1096/fj.14-256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Khoury J, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 65.Wyss-Coray T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 66.Chakrabarty P, et al. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J. Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 68.Rajmohan R, Reddy PH. Amyloid beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J. Alzheimers Dis. 2017;57:975–999. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butterfield DA, Boyd-Kimball D. Amyloid β-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14:426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krabbe G, et al. Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLOS ONE. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-β. J. Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Sanchez JL, et al. Plasma MCP-1 and changes on cognitive function in community-dwelling older adults. Alzheimer’s Res. Ther. 2022;14:5. doi: 10.1186/s13195-021-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee W-J, et al. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: A two-year follow-up study. Sci. Rep. 2018;8:1280. doi: 10.1038/s41598-018-19807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sokolova A, et al. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2008;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T, et al. MCP-1 levels in astrocyte-derived exosomes are changed in preclinical stage of Alzheimer’s disease. Front. Neurol. 2023 doi: 10.3389/fneur.2023.1119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, et al. IL-9 and Th9 cells in health and diseases—From tolerance to immunopathology. Cytokine Growth Factor Rev. 2017;37:47–55. doi: 10.1016/j.cytogfr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammed H, Salloom DF. Evaluation of interleukin-9 serum level and gene polymorphism in a sample of Iraqi type 2 diabetic mellitus patients. Meta Gene. 2021;27:100845. doi: 10.1016/j.mgene.2020.100845. [DOI] [Google Scholar]
- 78.Khalaf NF, et al. Pre-diabetes and diabetic neuropathy are associated with low serum levels of interleukin-9. Beni-Suef Univ. J. Basic Appl. Sci. 2023;12:75. doi: 10.1186/s43088-023-00412-6. [DOI] [Google Scholar]
- 79.Kuhn MK, et al. Alzheimer’s disease-specific cytokine secretion suppresses neuronal mitochondrial metabolism. bioRxiv. 2023 doi: 10.1101/2023.04.07.536014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wharton W, et al. Interleukin 9 alterations linked to Alzheimer disease in African Americans. Ann. Neurol. 2019;86:407–418. doi: 10.1002/ana.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werle M, Schmal U, Hanna K, Kreuzer J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc. Res. 2002;56:284–292. doi: 10.1016/S0008-6363(02)00600-4. [DOI] [PubMed] [Google Scholar]
- 82.Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-7 through NFκB and MAPK dependent pathways in rat astrocytes. Brain Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L-W, Tu Y-F, Huang C-C, Ho C-J. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflamm. 2012;9:175. doi: 10.1186/1742-2094-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 85.Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012;2012:1–15. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto T, Yamashita A, Yamada K, Hata R-I. Immunohistochemical localization of chemokine CXCL14 in rat hypothalamic neurons. Neurosci. Lett. 2011;487:335–340. doi: 10.1016/j.neulet.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 87.Tchelingerian JL, Vignais L, Jacque C. TNF alpha gene expression is induced in neurones after a hippocampal lesion. Neuroreport. 1994;5:585–588. doi: 10.1097/00001756-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Wood LB, et al. Identification of neurotoxic cytokines by profiling Alzheimer’s disease tissues and neuron culture viability screening. Sci. Rep. 2015;5:16622. doi: 10.1038/srep16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saleh A, et al. Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: Diminished expression in diabetes may contribute to sensory neuropathy. Brain Res. 2011;1423:87–95. doi: 10.1016/j.brainres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 90.DiLeonardi, A. M. Establishing a Protocol to Culture Primary Hippocampal Neurons. Decom Army Res. Lab. (2020).
- 91.Tomassoni-Ardori F, Hong Z, Fulgenzi G, Tessarollo L. Generation of functional mouse hippocampal neurons. Bio Protoc. 2020;10:e3702. doi: 10.21769/BioProtoc.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bandyopadhyay S. Role of neuron and glia in Alzheimer’s disease and associated vascular dysfunction. Front. Aging Neurosci. 2021 doi: 10.3389/fnagi.2021.653334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nordengen K, et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J. Neuroinflamm. 2019;16:46. doi: 10.1186/s12974-019-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aristyani S, Nur MI, Widyarti S, Sumitro SB. In silico study of active compounds ADMET profiling in Curcuma xanthorrhiza Roxb and Tamarindus indica as tuberculosis treatment. J. Jamu Indo. 2018;3:101–108. doi: 10.29244/jji.v3i3.67. [DOI] [Google Scholar]
- 95.Rizzari C, et al. Asparagine levels in the cerebrospinal fluid of children with acute lymphoblastic leukemia treated with pegylated-asparaginase in the induction phase of the AIEOP-BFM ALL 2009 study. Haematologica. 2019;104:1812–1821. doi: 10.3324/haematol.2018.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Page KA, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy EJ. Blood–brain barrier and brain fatty acid uptake: Role of arachidonic acid and PGE2. J. Neurochem. 2015;135:845–848. doi: 10.1111/jnc.13289. [DOI] [PubMed] [Google Scholar]
- 98.Kelly M, Widjaja-Adhi MAK, Palczewski G, von Lintig J. Transport of vitamin A across blood–tissue barriers is facilitated by STRA6. FASEB J. 2016;30:2985–2995. doi: 10.1096/fj.201600446R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richard DM, et al. L-Tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009;2:45–60. doi: 10.4137/IJTR.S2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fricker RA, Green EL, Jenkins SI, Griffin SM. The influence of nicotinamide on health and disease in the central nervous system. Int. J. Tryptophan Res. 2018;11:1178646918776658. doi: 10.1177/1178646918776658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaragozá R. Transport of amino acids across the blood-brain barrier. Front. Physiol. 2020;11:973. doi: 10.3389/fphys.2020.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J, et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: Metabolic basis for dementia. Sci. Rep. 2016;6:27524. doi: 10.1038/srep27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nonaka Y, et al. Lauric acid stimulates ketone body production in the KT-5 astrocyte cell line. J. Oleo Sci. 2016;65:693–699. doi: 10.5650/jos.ess16069. [DOI] [PubMed] [Google Scholar]
- 104.Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lodha D, Subramaniam JR. High fructose negatively impacts proliferation of NSC-34 motor neuron cell line. J. Neurosci. Rural Pract. 2022;13:114–118. doi: 10.1055/s-0041-1742120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q, Galleano M, Cederbaum AI. Cytotoxicity and apoptosis produced by arachidonic acid in hep G2 cells overexpressing human cytochrome P4502E1. J. Biol. Chem. 1997;272:14532–14541. doi: 10.1074/jbc.272.23.14532. [DOI] [PubMed] [Google Scholar]
- 107.Huck S, Grass F, Hatten ME. Gliotoxic effects of α-aminoadipic acid on monolayer cultures of dissociated postnatal mouse cerebellum. Neuroscience. 1984;12:783–791. doi: 10.1016/0306-4522(84)90170-2. [DOI] [PubMed] [Google Scholar]
- 108.Lu X, Li C, Wang Y-K, Jiang K, Gai X-D. Sorbitol induces apoptosis of human colorectal cancer cells via p38 MAPK signal transduction. Oncol. Lett. 2014;7:1992–1996. doi: 10.3892/ol.2014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vesprini ND, Spencer GE. Retinoic acid induces changes in electrical properties of adult neurons in a dose- and isomer-dependent manner. J. Neurophysiol. 2014;111:1318–1330. doi: 10.1152/jn.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silk JD, et al. IDO induces expression of a novel tryptophan transporter in mouse and human tumor cells. J. Immunol. 2011;187:1617–1625. doi: 10.4049/jimmunol.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffin SM, Pickard MR, Hawkins CP, Williams AC, Fricker RA. Nicotinamide restricts neural precursor proliferation to enhance catecholaminergic neuronal subtype differentiation from mouse embryonic stem cells. PLoS One. 2020;15:e0233477. doi: 10.1371/journal.pone.0233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bio-Rad. Bio-Plex Pro Assay 384-Well Protocol Bulletin 7269.
- 113.Fleeman RM, Kuhn MK, Chan DC, Proctor EA. Apolipoprotein E ε4 modulates astrocyte neuronal support functions in the presence of amyloid-β. J. Neurochem. 2023;165:536–549. doi: 10.1111/jnc.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hwang JJ, et al. The human brain produces fructose from glucose. JCI Insight. 2017;2:e90508. doi: 10.1172/jci.insight.90508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Generated data for analysis is included and available in the Supplementary Files of this article.
All code is publicly available at https://github.com/Brubaker-Lab/AD-T2D-Cytokine-Manuscript.