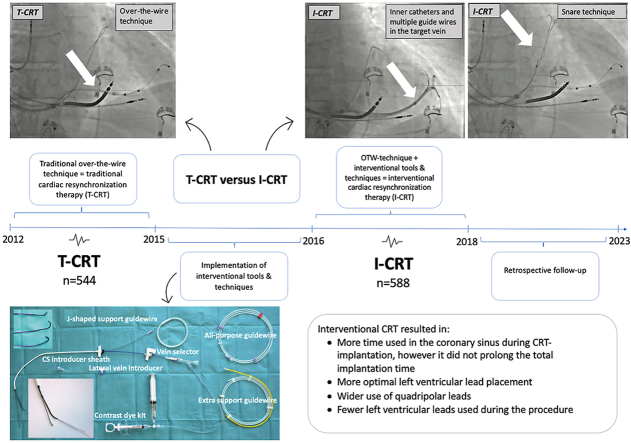
Abstract
Background
Interventional cardiac resynchronization therapy (I-CRT) for left ventricular lead (LVL) placement works as a supplement to traditional (over-the-wire) cardiac resynchronization therapy (T-CRT). It has been argued that I-CRT is a time-consuming and complicated procedure.
Objective
The purpose of this study was to investigate differences in procedure-related, perioperative, postoperative, and clinical endpoints between I-CRT and T-CRT.
Methods
This single-center, retrospective, cohort study included all consecutive patients receiving a CRT-pacemaker/defibrillator between January 1, 2012, and August 31, 2018. Patients underwent T-CRT from January 1, 2012, to June 1, 2015, and I-CRT from January 1, 2016, to August 31, 2018. We obtained data from patient record files, fluoroscopic images, and the Danish Pacemaker and ICD Register. Data were analyzed using Wilcoxon rank-sum/linear regression for continuous variables and the Pearson χ2/Fisher exact for categorical variables.
Results
Optimal LVL placement was achieved in 82.7% of the I-CRT group and 76.8% of the T-CRT group (P = .015). In the I-CRT group, 99.0% of LVLs were quadripolar vs 55.3% in the T-CRT group (P <.001). Two or more leads were used during the procedure in 0.7% and 10.5% of all cases in the I-CRT and T-CRT groups, respectively (P <.001). Total implantation time was 81.0 minutes in the I-CRT group and 83.0 minutes in the T-CRT group (P = .41). Time with catheters in the coronary sinus was 45.0 minutes for the I-CRT group vs 37.0 minutes in the T-CRT group, respectively (P <.001).
Conclusion
I-CRT did not prolong total implantation time despite longer time with catheters in the coronary sinus. I-CRT allowed more optimal LVL placement, wider use of quadripolar leads, and use of fewer leads during the procedure.
Keywords: Cardiac resynchronization therapy, Interventional cardiac resynchronization therapy, Traditional cardiac resynchronization therapy, Left ventricular pacemaker lead placement, Optimal left ventricular lead placement
Graphical abstract
Key Findings.
-
▪
Interventional cardiac resynchronization therapy (I-CRT) works as an add-on technique to the traditional cardiac resynchronization therapy (T-CRT) approach and offers more options for left ventricular lead placement, including better overview of the coronary sinus anatomy, flexible inner catheters, and multiple guidewires.
-
▪
I-CRT allows placement of more left ventricular leads in an optimal region of the left ventricle for biventricular pacing.
-
▪
The vast majority of the left ventricular leads placed with the I-CRT approach were quadripolar leads, and in only a few cases was >1 ventricular lead used during implantation.
-
▪
Although more time was used in the coronary sinus with the I-CRT approach, the total implantation time was not prolonged, suggesting that implanting physicians have become more experienced at identifying and accessing the ostium of the coronary sinus.
Introduction
Cardiac resynchronization therapy (CRT) is an important treatment for patients with advanced heart failure, reduced ejection fraction, and left bundle branch block.1 CRT is based on the principles of biventricular pacing, which aims to correct electromechanical dyssynchrony of the ventricles by endocardial pacing of the right ventricle and epicardial pacing of the left ventricle (LV).2 Epicardial pacing of the LV relies on placement of a pacing lead in one of the tributaries of the coronary sinus (CS).2,3 Previous studies have shown that CRT improves heart failure symptoms, cardiac function, and reduces mortality.1,4,5 Despite these findings, an estimated 30%–40% of patients receiving CRT do not respond to the treatment or even experience a clinical deterioration.6, 7, 8 These patients are considered nonresponders. Nonresponse is multifactorial, partly caused by insufficient biventricular pacing and suboptimal left ventricular lead (LVL) placement.8,9
Achieving a successful CRT response is mainly determined by the location of the LVL.10 Earlier observational studies have suggested LVL placement in the midportion or basal segment of a posterior or lateral wall of the LV improves clinical outcome compared to LVL placement in an apical or anterior region.6,10,11 Most patients eligible for CRT have left bundle branch block (LBBB), in which the site of latest activation typically is in the posterior or lateral wall of the LV. Therefore, targeting these regions results in restoration of coordinated myocardial contraction and thus increasing cardiac output and improving cardiac function.3
Several steps must be completed to achieve successful LVL placement, including localization and cannulation of the CS, creation of access to the target branch, and introduction of the LVL into the target branch.2,12,13 Delivery and placement of the LVL represents the most challenging step in CRT and may cause several obstacles for the implanting physician, especially in cases with anatomic abnormalities or variations.12, 13, 14 In 7.5%–10% of cases, LVL placement through the CS fails, possibly due to lack of operator experience in using traditional tools.2
A new approach, interventional cardiac resynchronization therapy (I-CRT), integrates new methods for CS cannulation and LVL placement.2,6,13,14 The I-CRT approach relies on a catheter-based system. This technique originally was invented by interventional cardiologists and radiologists. The traditional cardiac resynchronization therapy (T-CRT) approach is restricted to the conventional over-the-wire technique. A mainstay of I-CRT is the ability to render better support in the CS when placing the LVL by using dedicated inner catheters with a far longer and deeper reach in the target branch compared to the inner catheters provided with the delivery systems from the lead manufacturers.2,6,12 It is important to emphasize that the I-CRT approach is not a new CRT method. I-CRT works as an add-on-approach to T-CRT and provides new tools and techniques to the over-the-wire technique.
To date, the use of I-CRT has been only sparsely examined. Therefore, evidence supporting I-CRT as the standard of care method for implanting LVL in CRT is limited. Knowledge of interventional tools and techniques have been described in the literature,2,12, 13, 14 but only one study investigated differences between I-CRT and T-CRT.6 Therefore, this study aimed to assess differences in procedure-related endpoints, perioperative and postoperative endpoints, and clinical endpoints between the I-CRT approach and the T-CRT approach.
Materials and methods
Study design
This retrospective cohort study included all consecutive patients who received a CRT-pacemaker/defibrillator (CRT-P/D) between January 1, 2012, and August 31, 2018, at Odense University Hospital (Odense, Denmark). Between January 1, 2012, and June 1, 2015, all patients had the LVL implanted using the T-CRT approach. In June 2015, I-CRT was introduced. From June 2015 to January 1, 2016, the implanting physicians were trained in I-CRT tools and techniques, so patients receiving CRT during this period were excluded. From January 1, 2016, to August 31, 2018, all patients had the LVL implanted using the I-CRT approach. All I-CRT systems were from Merit Medical. A total of 1142 patients were included in the study. The study was approved by the DPIR Steering Committee, the Regional Council of Southern Denmark, and the Region of Southern Denmark Register of Research Projects. Approval from the Ethics Committee was not required for registry-based studies according to Danish law.
Patient population
Patients were considered eligible if they were ≥18 years of age and were in New York Heart Association (NYHA) functional class II–IV despite receiving optimal medical therapy, and had LV ejection fraction ≤35% and LBBB with QRS ≥130 ms. Some patients had QRS >150 ms (irrespective of the bundle branch configuration) or chronic right ventricular pacing. These inclusion criteria were considered as the classic inclusion criteria, and patients who met these criteria were considered classic CRT patients. Patients also were considered eligible if they already had a pacing indication, heart failure, and an expected high burden of right ventricular pacing. These patients were considered nonclassic CRT patients. Both de novo implants and upgrades from previous devices were included. Patients on the waiting list for a heart transplant or LV assist device were excluded.
Materials
All patients who received a CRT-P/D during the study period were registered in the Danish Pacemaker and ICD Register (DPIR). DPIR holds detailed clinical and technical information on all cardiac implantable electronic device procedures in Denmark.15 Supplementary data from DPIR were cross-linked with data obtained from patient record files and fluoroscopic images using the unique personal identifier (CPR) assigned to all Danish citizens.16
Endpoints
Our primary endpoint was procedural durations: time used in the CS and total implantation time. Time used in the CS was calculated from the first fluoroscopy identifying the CS ostium to the last fluoroscopy with the LVL in place. LVL placement were assessed clockwise in the left anterior oblique view and categorized as anterior (≤2 o’clock), lateral (3–4 o’clock), or posterior (>4 o’clock). Correspondingly, LVL placement was assessed as apical, midventricular, or basal in the right anterior oblique view.10 Optimal LVL placement was defined as posterior/lateral and basal/midventricular.6
Secondary endpoints are perioperative and postoperative endpoints and clinical endpoints. At 3-month follow-up, a CRT responder was defined as a patient who experienced a clinical improvement in functional capacity and heart failure symptoms as assessed by an overall evaluation. In some cases, echocardiography was performed and included in the evaluation. Clinical endpoints were only assessed for classic CRT patients (Figure 1).
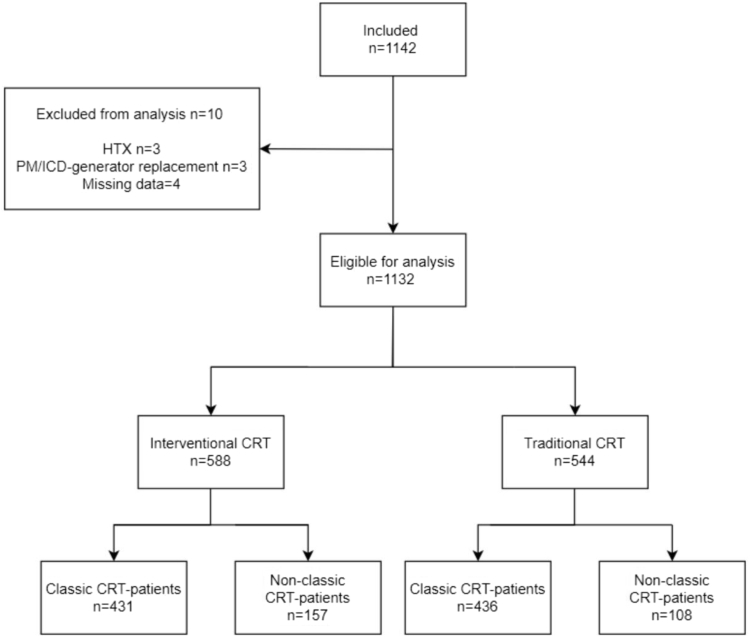
Figure 1.
Flowchart showing inclusion and exclusion of patients eligible for the study. CRT = cardiac resynchronization therapy; HTX = heart transplant; ICD = implantable cardioverter-defibrillator; PM = pacemaker.
Statistical analysis
Categorical variables are given as percentage or frequency. Continuous variables are given as median [interquartile range]. Differences were assessed using Wilcoxon rank-sum and linear regression for continuous variables. Linear regression was only used to estimate relative procedure durations. The Pearson χ2 and Fisher exact tests were used to assess differences in categorical variables. Two-sided P <.05 was considered significant. All analyses were performed using Stata Statistical Software Release 17 (StataCorp, College Station, TX)
Results
Patient and baseline characteristics
Of the 1142 patients included in the study, 1132 were eligible for analysis; 588 patients underwent I-CRT and 544 patients underwent T-CRT (Figure 1). Patients in the I-CRT group were older (median 72.0 years; P <.001) and were in a lower NYHA class compared to patients in the T-CRT group (P <.001). More patients in the I-CRT group received a CRT-P device than patients in the T-CRT group (51.7% vs 41.7%, respectively; P <.001). Most procedures were de novo implantations (I-CRT 74.1% vs T-CRT 75.9%). The remaining procedures were upgrades. Baseline characteristics are given in Table 1.
Table 1.
Patient characteristics
| Factor | Level | T-CRT | I-CRT | P value |
|---|---|---|---|---|
| No. | 544 | 588 | ||
| Sex | Female | 111 (20.4) | 144 (24.5) | .10 |
| Male | 433 (79.6) | 444 (75.5) | ||
| Age (y) | 69.0 [62.0–76.0] (n = 544) | 72.0 [64.0–78.0] (n = 588) | <.001 | |
| QRS morphology | Narrow QRS | 54 (10.0) | 85 (14.5) | .049 |
| Paced QRS | 102 (18.9) | 129 (22.0) | ||
| LBBB | 284 (52.5) | 270 (46.0) | ||
| RBBB | 41 (7.6) | 35 (6.0) | ||
| Wide QRS other than LBBB/RBBB | 60 (11.1) | 68 (11.6) | ||
| QRS width (ms) | 160.0 [142.0–176.0] (n = 515) | 158.0 [138.0–174.0] (n = 585) | .27 | |
| NYHA functional class | I | 9 (2.4) | 6 (1.4) | <.001 |
| II | 210 (56.5) | 316 (75.2) | ||
| III | 150 (40.3) | 96 (22.9) | ||
| IV | 3 (0.8) | 2 (0.5) | ||
| Device type | CRT-P | 227 (41.7) | 304 (51.7) | <.001 |
| CRT-D | 317 (58.3) | 284 (48.3) | ||
| Procedure type | De novo | 413 (75.9) | 436 (74.1) | .49 |
| Upgrades | 131 (24.1) | 152 (25.9) |
Values are n (%) or median [interquartile range] unless otherwise indicated.
CRT-D = cardiac resynchronization therapy–defibrilator; CRT-P = cardiac resynchronization therapy–pacemaker; I-CRT = interventional cardiac resynchronization therapy; LBBB = left bundle branch block; NYHA = New York Heart Association; RBBB = right bundle branch block; T-CRT = traditional cardiac resynchronization therapy.
Procedural durations
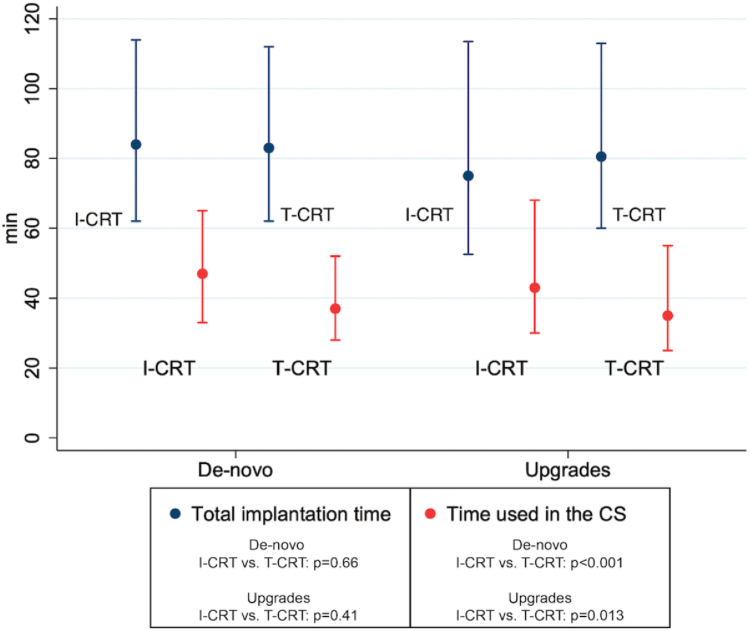
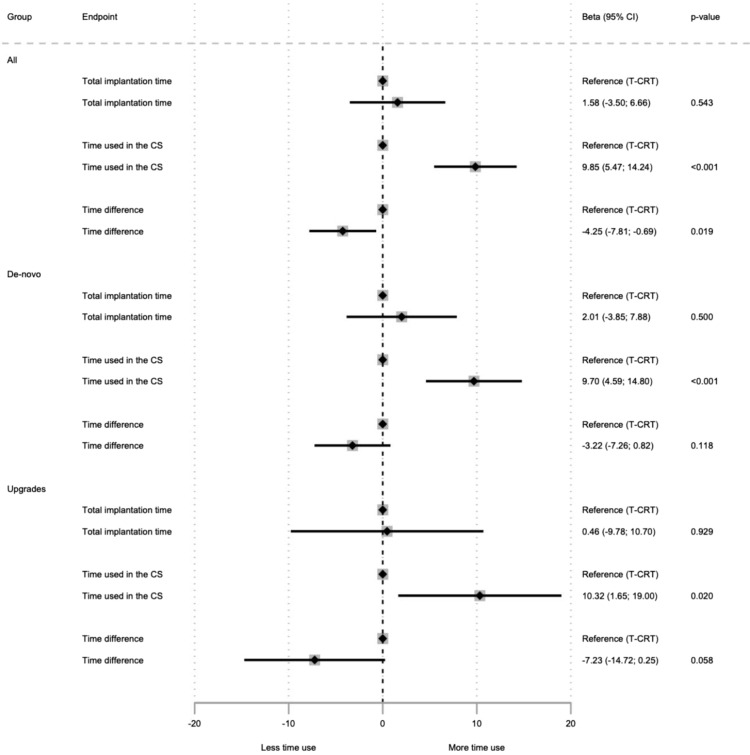
Time used in the CS was longer in the I-CRT group compared to the T-CRT group (45.0 vs 37.0 minutes; P <.001) despite no overall difference in total implantation time (I-CRT 81.0 vs T-CRT 83.0 minutes; P = .41). Similar results were found after stratifying for procedure type (de novo vs upgrades) with longer time duration in the CS in the I-CRT group despite no difference in total implantation time. Stratified results are shown in Figures 2 and 3.
Figure 2.
Total implantation time and time used in the coronary sinus (CS) for the interventional cardiac resynchronization therapy (I-CRT) and traditional cardiac resynchronization therapy (T-CRT) groups for de novo implantations (n = 849; I-CRT 436, T-CRT 413) and upgrades (n = 283; I-CRT 152, T-CRT 131).
Figure 3.
Forest plot of relative procedural durations for the interventional cardiac resynchronization therapy and traditional cardiac resynchronization therapy (T-CRT) groups for all implantations (n = 1132), de novo implantations (n = 841), and upgrades (n = 278). Time difference represents the difference between total implantation time and time used in the coronary sinus (CS). Thirteen patients were excluded from the analysis due to unavailable data. CI = confidence interval
LVL placement and type
The LVL was primarily placed laterally or midventricular for both the I-CRT and T-CRT groups. In the I-CRT group, 85.8% of all LVLs were placed laterally vs 82.8% in the T-CRT group (P = .11). Moreover, in the I-CRT group LVLs were more often placed mid-ventricularly compared to the T-CRT group (69.9% vs 58.6%; P <.001). In total, 82.7% of all LVLs were placed optimally in the I-CRT group compared to 76.8% in the T-CRT group (P = .015). The remaining LVLs were placed suboptimally. Quadripolar leads were almost exclusively used in the I-CRT group (99.0%) compared to only 55.3% in the T-CRT group (P <.001). Furthermore, the total number of LVLs used during the procedure were lower in the I-CRT group (≥2 LVLs: 0.7% vs 10.5%; P <.001). LVL placements are listed in Table 2.
Table 2.
Procedure-related endpoints
| Factor | Level | T-CRT | I-CRT | P value |
|---|---|---|---|---|
| No. | 544 | 588 | ||
| LVL placement (LAO) | Anterior | 64 (12.2) | 49 (8.5) | .11 |
| Lateral | 434 (82.8) | 497 (85.8) | ||
| Posterior | 26 (5.0) | 33 (5.7) | ||
| LVL placement (RAO)n (%) | Apical | 42 (7.8) | 42 (7.2) | <.001 |
| Basal | 182 (33.6) | 133 (22.9) | ||
| Mid | 317 (58.6) | 406 (69.9) | ||
| Optimal LVL placement | Optimal | 418 (76.8) | 486 (82.7) | .015 |
| LVL type | Bipolar | 243 (44.7) | 6 (1.0) | <.001 |
| Quadripolar | 301 (55.3) | 582 (99.0) | ||
| LVLs used | 1 | 487 (89.5) | 584 (99.3) | <.001 |
| ≥2 | 57 (10.5) | 4 (0.7) | ||
| Snare technique used | Yes | 1 (0.2) | 89 (15.1) | <.001 |
| Inner catheter used | Yes | 91 (16.7) | 410 (69.7) | <.001 |
| Inner catheter in target branch | Yes | 18 (3.3) | 398 (67.7) | <.001 |
| Venoplasty of the subclavian vein/superior vena cava | Yes | 1 (0.2) | 10 (1.7) | .012 |
| Venoplasty in the CS | Yes | 0 (0.0) | 13 (2.2) | <.001 |
| X-ray duration (min) | 15.0 [9.0–24.0] (n = 543) | 16.0 [10.0–26.0] (n = 588) | .032 |
Values are given as n (%) or median [interquartile range] unless otherwise indicated.
CS = coronary sinus; LAO = left anterior oblique; LVL = left ventricular lead; RAO = right anterior oblique; other abbreviations as in Table 1.
Procedure-related endpoints
Snare technique was used more often in the I-CRT group than the T-CRT group (15.1% vs 0.2%, respectively; P <.001). Inner catheters were used more often in the I-CRT group than the T-CRT group (69.7% vs 16.7%; P <.001). In particular, inner catheters in a target branch were used often in the I-CRT group and in only a few cases in the T-CRT group (67.7% vs 3.3%, respectively; P <.001). Venoplasty of the superior vena cava or the subclavian vein was used more often in the I-CRT group than the T-CRT group (1.7% vs 0.2%, respectively; P <.001). Similarly, venoplasty in the CS was used only in the I-CRT group compared to the T-CRT group (2.2% vs 0.0%, respectively; P <.001). X-ray duration was slightly longer in the I-CRT group than the T-CRT group (16.0 vs 15.0 minutes; P = .032). Procedure-related endpoints are listed in Table 2.
Perioperative and postoperative complications
Dissections in the CS were rare but occurred more frequently in the I-CRT group compared to the T-CRT group (4.1% vs 1.7%, respectively; p<0.05). There were no differences in LVL displacement (1.4% vs 1.1%; P = .70) and infection rate (2.0% vs 2.8%; P = .43) between the I-CRT and T-CRT groups, respectively. Similarly, phrenic nerve stimulation occurred equally in the I-CRT and T-CRT groups (6.8% vs 7.0%, respectively; P = .90). Perioperative and postoperative complications are listed in Table 3.
Table 3.
Perioperative and postoperative endpoints
| Factor | Level | T-CRT | I-CRT | P value |
|---|---|---|---|---|
| No. | 544 | 588 | ||
| Dissection of vein(s) | Yes | 9 (1.7) | 24 (4.1) | .015 |
| LVL displacement | Yes | 6 (1.1) | 8 (1.4) | .70 |
| Infection | Yes | 15 (2.8) | 12 (2.0) | .43 |
| Phrenic nerve stimulation | Yes | 38 (7.0) | 40 (6.8) | .90 |
Values are given as n (%) unless otherwise indicated.
I-CRT = interventional cardiac resynchronization therapy; LVL = left ventricular lead; T-CRT = traditional cardiac resynchronization therapy.
Clinical endpoints
Only classic CRT patients were eligible for analysis of clinical endpoints. In the I-CRT group, 68.3% of subjects were responders, whereas 71.0% of subjects were responders in the T-CRT group (P = .40). Statistically, the number of patients hospitalized due to heart failure ≥2 times did not differ between the groups (I-CRT 6.7% vs T-CRT 3.8%; P = .13). Clinical endpoints are listed in Table 4.
Table 4.
Clinical endpoints
| Factor | Level | T-CRT | I-CRT | P value |
|---|---|---|---|---|
| No. | 436 | 431 | ||
| CRT responder | Responder | 277 (71.0) | 271 (68.3) | .40 |
| Hospitalizations (24 mo after implant) | None | 378 (88.9) | 357 (85.0) | .13 |
| Once | 31 (7.3) | 35 (8.3) | ||
| Twice or more | 16 (3.8) | 28 (6.7) |
Values are given as n (%) unless otherwise indicated.
CRT = cardiac resynchronization therapy; I-CRT = interventional cardiac resynchronization therapy; T-CRT = traditional cardiac resynchronization therapy.
Discussion
We investigated differences between the I-CRT and T-CRT approaches for implantation of LVL in CRT. We found that use of I-CRT did not prolong the total implantation time despite more time used in the CS. Moreover, I-CRT allowed for wider usage of different techniques (snare technique, venoplasty, and inner catheters), resulting in greater use of quadripolar leads with better LVL placement despite use of fewer leads during the procedure. Only limited differences in perioperative, postoperative, and clinical endpoints were observed.
I-CRT works as an add-on approach to T-CRT and allows new options for LVL placement. However, techniques for LVL placement are poorly described in the literature and have not been studied systematically. This creates a paradox with an increasing usage of I-CRT despite the lack of evidence supporting its beneficial value. Most of the publications on I-CRT are reviews describing how to perform the I-CRT approach. Only one study assessed differences in the use of I-CRT and T-CRT.6 I-CRT has been criticized for being a more time-consuming procedure despite limited knowledge on differences in time consumption between I-CRT and T-CRT. However, I-CRT has been implemented as part of the invasive treatment in patients with heart failure, so usage of I-CRT should be examined.
Time used in the CS was significantly longer using I-CRT compared to T-CRT in our study. Sperzel et al17 found a similar result for time used in the CS of 33 ± 47 minutes with the over-the-wire technique. Despite more time spent in the CS with I-CRT, we observed no differences in total implantation time. Therefore, we assume that the tools for identifying and introducing catheters into the CS are better with I-CRT.
Conduction system pacing in terms of His-bundle pacing (HBP) or left bundle branch area pacing (LBBAP) has been advocated as a new and safe alternative to CRT.18 The largest randomized trial comparing HBP and CRT performed by Vinther et al19 showed procedural times of 137 ± 46 minutes using HBP compared to 102 ± 34 minutes using CRT. Furthermore, a retrospective study investigating LBBAP in patients eligible for CRT performed by Vijayaraman et al20 found procedural times of 105 ± 54 minutes using LBBAP. Moreover, fluoroscopy times for HBP (22.0 ± 14.0 minutes) and LBBAP (19.0 ± 15.0 minutes) are considerably longer compared to our findings but still shorter than the fluoroscopy times reported in the study by Jackson et al6 (I-CRT 29.6 minutes vs T-CRT 41.9 minutes). It could be argued that procedural and fluoroscopy times are longer using HBP and LBBAP due to less experience with the procedure. However, in our study we observed remarkably shorter CRT implantation times and fluoroscopy times compared to Vinther et al19 and Vijayaraman et al.20
We found that 82.7% of LVLs were placed optimally in the I-CRT group compared to 76.8% in the T-CRT group. It is important that the LVL is placed in an optimal region of the LV to achieve the best resynchronization. Most eligible CRT patients have LBBB in which the site of latest activation usually is in the mid/basal ventricular segment of a lateral/posterior wall of the LV. A randomized controlled trial found that LVL placement in the latest activated sites (mainly the lateral/posterior wall) was associated with better CRT responses.11 Jackson et al6 defined optimal LVL placement as placement in the mid/basal segment of the lateral/posterior wall of the LV. Using this definition, Jackson et al6 found that 87.0% LVLs were placed optimally using I-CRT vs 75.0% using T-CRT. We used the same definition as Jackson et al6 to assess optimal LVL placement. Despite more LVLs being placed optimally in the I-CRT group, we found no differences in clinical endpoints. The majority of I-CRT patients were in NYHA functional class II, and more T-CRT patients were in NYHA functional class III. Therefore, improving the clinical outcome for I-CRT patients was difficult to prove because the patients already were in a lower NYHA functional class.
In some cases the implanting physician must change the LVL during the procedure because of difficult anatomy or circumstances relating to the LVL design, such as the shape of the distal part of the lead. Therefore, the types and numbers of LVLs used during the procedure are of great interest. Sperzel et al17 found that 78.0% of all LVLs were placed in the first-choice target vein using the over-the-wire technique. Compared to our study, ≥2 leads were used in 0.7% and 10.5% of all cases in the I-CRT and T-CRT groups, respectively. Only 1 lead was used in 99.3% and 89.5% of all cases in the I-CRT and T-CRT groups, respectively. It is not an ideal comparison between our study and that of Sperzel et al17 because the implanting physicians in our study did not always switch the target vein but continued in the first-choice target vein with a new LVL.
Quadripolar leads were available during the entire study period. Overall, fewer quadripolar leads were used in the T-CRT group, probably because of difficulties in placing the lead sufficiently deep into the target vein to accommodate all 4 poles of the lead. Previous studies by Sperzel et al17 and Tomassoni et al21 indicate that use of quadripolar leads may reduce the number of patients experiencing phrenic nerve stimulation (PNS) due to more programming options of the pacing vector. Therefore, we expected fewer cases with PNS in the I-CRT group due to extensive use of quadripolar leads. However, we did not find fewer cases of PNS in the I-CRT group. Sperzel et al17 found that 11% suffered from PNS 1 month post CRT implantation. Compared to our findings, 6.8% and 7.0% suffered from PNS in the I-CRT and T-CRT groups, respectively. An advantage of our study is that we evaluated PNS 3–6 months post CRT implantation, which allowed us to evaluate the effect from the LVL after longer follow-up.
Displacement of the LVL is another postoperative risk. Behar et al22 showed that 1.7% of quadripolar leads vs 4.6% of bipolar leads displaced. With our wide use of quadripolar leads during the I-CRT period, we expected that fewer LVLs would displace compared to leads placed during the T-CRT period. However, we found that almost no leads displaced regardless of implantation technique. Even though more CS dissections occurred in the I-CRT group, all CRT implantations succeeded due to the ability to use inner catheters and multiple guidewires. None of the CS dissections caused any clinical complications, such as CS perforations, cardiac tamponade, or need for pericardial drainage.
In our study, fluoroscopy times were 16.0 minutes and 15.0 minutes for the I-CRT and T-CRT groups, respectively. Comparing our results to those of Jackson et al,6 we found remarkably shorter fluoroscopy times. Jackson et al6 were the first to assess differences between I-CRT and T-CRT. They reported fluoroscopy times of 29.6 minutes for the I-CRT group compared to 41.9 minutes for the T-CRT group. A possible explanation is that the study by Jackson et al6 was performed in an earlier period when the implanting physicians were less experienced. Jackson et al6 did not report total implantation times; rather, they used their fluoroscopy times as a surrogate for their procedural times.
Finally, we did not find any differences in clinical endpoints between patients receiving I-CRT and T-CRT. Considerable differences in NYHA functional class and age were observed at baseline, which minimized our ability to compare clinical endpoints. This could explain why we did not observe any differences in clinical endpoints between the 2 groups. Therefore, our clinical endpoints should be interpreted with caution. The definition of a CRT responder is not optimal but due to the retrospective study design the possibilities of assessing a clinical outcome was limited.
Study limitations
We present nonrandomized, retrospective, observational data from a single-center study. This allows us only to draw associations between our findings but no definite conclusions. In particular, conclusions on clinical outcomes should be interpreted with caution because patients were not comparable at baseline. Information on comorbidities may explain some of this, but no data on comorbidity or information on LV ejection fraction were included due to nonsystematic performances of echocardiography at follow-up. Our main focus were procedure-related endpoints; however, we did include clinical endpoints because evidence on clinical endpoints in I-CRT are just as limited as evidence on procedure-related endpoints. Therefore, LV ejection fraction and supplementary echocardiographic measures would be preferred as clinical outcomes in future studies. Additionally, it was only possible to assess time used in the CS in a subset of cases included in the T-CRT group due to missing fluoroscopic images. Only a few fluoroscopic images were missing during the I-CRT period. No data on the amount of contrast was included, only information on X-ray duration. Finally, we included consecutive patients who were registered in the DPIR, which minimized the risk of selection bias.
Conclusion
We found that use of I-CRT did not prolong total implantation time despite more time used in the CS. Furthermore, I-CRT resulted in more optimal LVL placement, wider use of quadripolar leads, and use of fewer LVLs during the procedure. More studies are needed to assess differences in perioperative, postoperative, and clinical endpoints.
Acknowledgments
Data-management, statistical supervision, OPEN-Analyse, and REDCap electronic data capture tools were hosted by OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark.
Funding Sources
Dr Jakobsen received a grant from Odense University Hospital, J.B. Winsløws Vej 4, 5000 Odense C (Grant Number A5255).
Disclosures
Dr Johansen reports personal fees from Medtronic (consultant) and institutional fees from Merit Medical. Dr Sandgaard reports institutional fees from Merit Medical. All other authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patient consent was not required for this study.
Ethics Statement
The study was approved by the DPIR Steering Committee, the Regional Council of Southern Denmark, and the Region of Southern Denmark Register of Research Projects. Approval from the Ethics Committee was not required for registry-based studies according to Danish law.
Data Availability
Data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1.Cleland J.G., Daubert J.C., Erdmann E., et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Johansen J.B., Nielsen J.C., Kristensen J., Sandgaard N.C. Troubleshooting the difficult left ventricular lead placement in cardiac resynchronization therapy: current status and future perspectives. Expert Rev Med Devices. 2022;19:341–352. doi: 10.1080/17434440.2022.2075728. [DOI] [PubMed] [Google Scholar]
- 3.Jarcho J.A. Biventricular pacing. N Engl J Med. 2006;355:288–294. doi: 10.1056/NEJMct055185. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg I., Kutyifa V., Klein H.U., et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt J.D., Wilkoff B.L. Interventional electrophysiology and cardiac resynchronization therapy: delivering electrical therapies for heart failure. Circulation. 2007;115:2208–2220. doi: 10.1161/CIRCULATIONAHA.106.655712. [DOI] [PubMed] [Google Scholar]
- 6.Jackson K.P., Hegland D.D., Frazier-Mills C., et al. Impact of using a telescoping-support catheter system for left ventricular lead placement on implant success and procedure time of cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2013;36:553–558. doi: 10.1111/pace.12103. [DOI] [PubMed] [Google Scholar]
- 7.Zou F., Brar V., Worley S.J. Interventional device implantation, part i: basic techniques to avoid complications: a hands-on approach. J Cardiovasc Electrophysiol. 2021;32:523–532. doi: 10.1111/jce.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq C., Burri H., Curnis A., et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE-CRT MPP) study: results from Phase I. Eur Heart J. 2019;40:2979–2987. doi: 10.1093/eurheartj/ehz109. [DOI] [PubMed] [Google Scholar]
- 9.Lubitz S.A., Singh J.P. Biventricular pacing: more is better. Eur Heart J. 2015;36:407–409. doi: 10.1093/eurheartj/ehu347. [DOI] [PubMed] [Google Scholar]
- 10.Kronborg M.B., Johansen J.B., Riahi S., et al. An anterior left ventricular lead position is associated with increased mortality and non-response in cardiac resynchronization therapy. Int J Cardiol. 2016;222:157–162. doi: 10.1016/j.ijcard.2016.07.235. [DOI] [PubMed] [Google Scholar]
- 11.Khan F.Z., Virdee M.S., Palmer C.R., et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Worley S.J. Challenging implants require tools and techniques not tips and tricks. Card Electrophysiol Clin. 2019;11:75–87. doi: 10.1016/j.ccep.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Worley S., Ellenbogen K.A. Application of interventional procedures adapted for device implantation: new opportunities for device implanters. Pacing Clin Electrophysiol. 2007;30:938–941. doi: 10.1111/j.1540-8159.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 14.Worley S.J. CRT delivery systems based on guide support for LV lead placement. Heart Rhythm. 2009;6:1383–1387. doi: 10.1016/j.hrthm.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Olsen T., Jørgensen O.D., Nielsen J.C., et al. Risk factors for cardiac implantable electronic device infections: a nationwide Danish study. Eur Heart J. 2022;43:4946–4956. doi: 10.1093/eurheartj/ehac576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 17.Sperzel J., Dänschel W., Gutleben K.J., et al. First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace. 2012;14:365–372. doi: 10.1093/europace/eur322. [DOI] [PubMed] [Google Scholar]
- 18.Burri H., Jastrzebski M., Cano O., et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS) Europace. 2023;25:1208–1236. doi: 10.1093/europace/euad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinther M., Risum N., Svendsen J.H., Møgelvang R., Philbert B.T. A randomized trial of His pacing versus biventricular pacing in symptomatic HF patients with left bundle branch block (His-alternative) JACC Clin Electrophysiol. 2021;7:1422–1432. doi: 10.1016/j.jacep.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Vijayaraman P., Ponnusamy S., Cano Ó., et al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol. 2021;7:135–147. doi: 10.1016/j.jacep.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Tomassoni G., Baker J., Corbisiero R., et al. Postoperative performance of the Quartet® left ventricular heart lead. J Cardiovasc Electrophysiol. 2013;24:449–456. doi: 10.1111/jce.12065. [DOI] [PubMed] [Google Scholar]
- 22.Behar J.M., Bostock J., Zhu Li A.P., et al. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol. 2015;26:540–546. doi: 10.1111/jce.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying this article will be shared on reasonable request to the corresponding author.