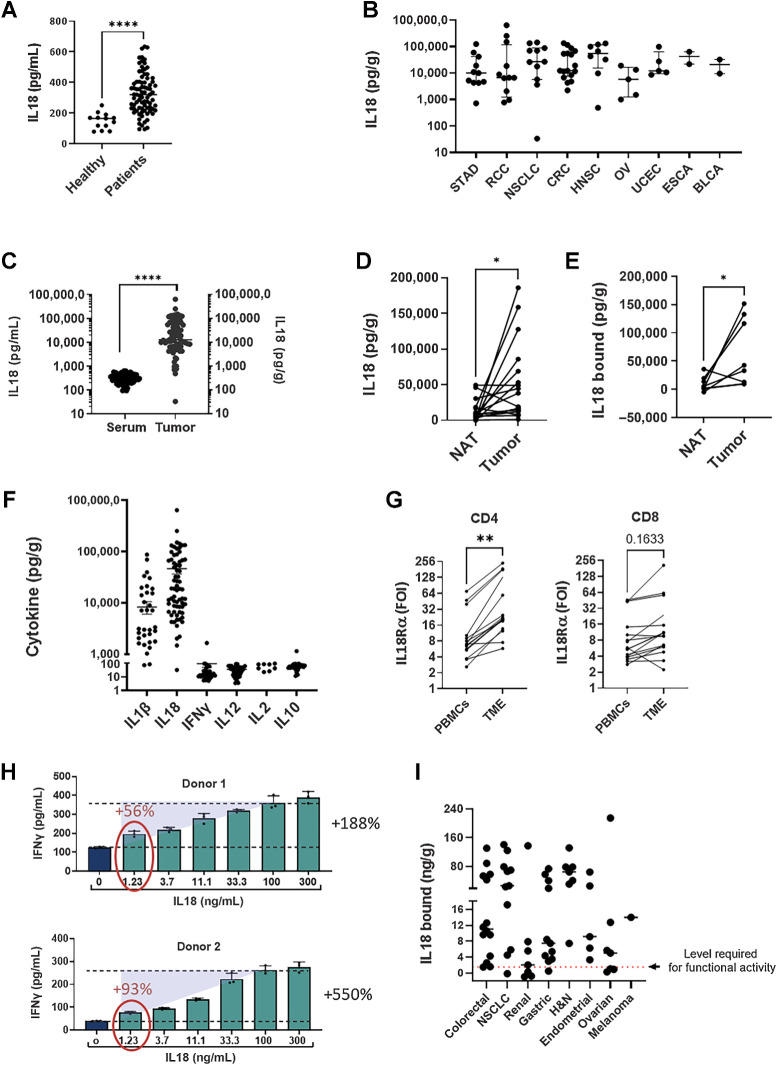
Figure 2.
IL18 is upregulated in patients with cancer and in the TME and mostly inactive. Tumor biopsies, normal tissues adjacent to the tumors (NAT) and serum samples from patients with cancer and healthy donors were analyzed. Tumor biopsies and NATs were dissociated, and supernatant was collected. IL18 and cytokine expression were analyzed using ELISA, CBA, and LEGENDplex kits. A, IL18 levels in sera from patients with cancer (n = 81) and healthy donors(n = 13). B, IL18 expression in tumor-derived supernatants (TDS) from individual patients across indications (n = 75). C, IL18 expression in sera and TDS. D, IL18 expression in matched NAT and tumor-derived supernatant samples (n = 17). E, Levels of IL18BP-bound IL18 in matched NAT and tumor-derived supernatant samples (n = 7). IL18BP-bound IL18 levels (n = 65) were calculated by deducting IL18 free from total IL18 measured for each sample by two separate ELISA assays. F, Cytokine expression in TDS (n = 56) were analyzed using CBA TH1/TH2/TH17 and LEGENDplex Human Inflammation Panel 1 kits. G, T cells were purified from tumor biopsies and matched PBMC (n = 16) and stained for IL18Ra expression by flow cytometry. H,Ex vivo stimulated human melanoma CD8+ TILs (n = 2) were co-cultured with melanoma antigen-expressing MEL624 cells in the presence of rIL18 (0–300 ng/mL) for 24 hours, after which supernatants were analyzed for IFNγ secretion. I, Levels of bound IL18 in tumor derived supernatants across indications. Tumor biopsies were dissociated, and supernatants were collected and analyzed using free and total IL18 ELISA assays. IL18BP-bound IL18 levels were calculated by deducting IL18 free from total IL18 measured for each sample by two separate ELISA assays. Dashed red line represents the level required for functional activity (1.2 ng/g). Each dot represents 1 patient. The median is depicted by a short black line. *, P < 0.05; **, P < 0.01; ***, P < 0.0001 by two-tailed t test or Mann–Whitney test.